Acetophenone: Past, Progress, and Prospects
Historical Development
Acetophenone first showed up in the chemical literature back in the early 1800s, starting its journey as a mysterious byproduct found in coal tar. Early chemists struggled to nail down its role, but curiosity about fragrant compounds and advances in coal chemistry brought it into focus. By the end of the 19th century, researchers like August G. Mitscherlich worked out better methods for making the compound, helping turn it from a lab accident into an industrial workhorse. Its mild, sweet aroma caught attention in fragrance circles, soon landing acetophenone in the growing world of synthetic perfumes and later, in pharmaceuticals and solvents. Acetophenone’s gradual climb from an odd lab sample to a staple of large-scale organic chemistry says a lot about how chemical knowledge spreads and transforms over time.
Product Overview
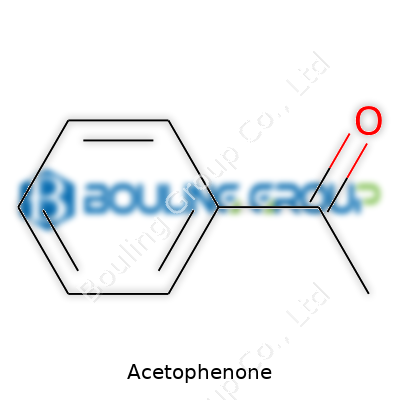
Acetophenone, with the chemical formula C8H8O, appears as a colorless liquid with a pleasant, sweet, and slightly floral odor, which has found uses from fragrance creation to manufacturing specialty solvents. It acts as a building block for several chemical syntheses. Over the years, the product has reached global commerce, manufactured in ton-scale for various industries including flavors, fragrances, pharmaceuticals, and even as an intermediate for polymers.
Physical & Chemical Properties
Acetophenone boils at about 202°C, and its melting point sits near 20°C, so it stays liquid under room temperature in most climates. The density checks in at roughly 1.03 g/ml, which makes sampling and storage straightforward using standard glassware. Its low water solubility means it tends to partition into organic phases, a trait valuable for extraction in processing. Acetophenone offers moderate stability and doesn’t degrade or polymerize under normal storage. Its reactivity centers on the carbonyl group: it can take part in reductions, oxidations, and a wide spectrum of condensation reactions. Recognizing these properties sets the foundation for effective handling and safe storage practices.
Technical Specifications & Labeling
Bulk producers tend to market acetophenone at a purity higher than 99%, which proves essential for downstream synthesis. Bottles and drums should bear proper hazard codes, including flammability and health risk indicators compliant with the Globally Harmonized System (GHS). Labels also outline batch numbers, manufacturer information, and recommended storage conditions: keep it cool, dry, and away from sources of ignition. Technical data sheets often list boiling/melting points, refractive index, and spectroscopic signatures, guiding quality control and troubleshooting during industrial processing. For labs and industrial users, those technical sheets aren’t just legal requirements, but practical guides for day-to-day safety and compliance.
Preparation Method
Friedel–Crafts acylation dominates commercial production of acetophenone. The typical route starts with benzene and acetyl chloride (or acetic anhydride), using an aluminum chloride catalyst to tie everything together. This method remains popular thanks to its reliability, relatively high yield, and compatibility with the established chemical supply chain. Older routes once included oxidation of ethylbenzene, but stricter safety and environmental controls made direct acylation more attractive. Academic labs sometimes make acetophenone using alternative oxidations or biotransformation, seeking greener chemistry or tighter selectivity, yet industry sticks close to the old Friedel–Crafts pathway because it’s robust on a large scale.
Chemical Reactions & Modifications
Acetophenone serves as a prime candidate for classic organic transformations. Its α-hydrogen enables it to undergo a range of aldol condensations and Michael additions, laying the groundwork for more complex pharmaceuticals and fragrance ingredients. Catalytic hydrogenation of acetophenone reduces the carbonyl, forming 1-phenylethanol, an ingredient used in both flavors and drug synthesis. Under oxidation, the methyl group adjacent to the carbonyl can transform into benzoic acid derivatives. Acetophenone also participates in the synthesis of heterocycles and other medicinally relevant intermediates by reacting with a spectrum of nucleophiles and electrophiles. Time in the lab with this compound gives real appreciation for its versatility—there’s always a new way to tweak its skeleton and give rise to fresh compounds that end up in the most unexpected products.
Synonyms & Product Names
Many chemists know acetophenone by its alternate names, such as phenyl methyl ketone or methyl phenyl ketone. In the flavor and fragrance circles, some professionals refer to it as Hypnone, a trade name still used by certain suppliers. These aliases matter in both regulatory filings and supply chain management: precise identification helps prevent mislabeling and mishaps, especially in global trade when one warehouse calls it acetophenone, another logs it under an older synonym, and a third gets shipment labeled “1-Phenylethanone.”
Safety & Operational Standards
Even though acetophenone feels familiar in labs and factories, standard safety precautions should never be skipped. It’s flammable and can cause skin and eye irritation, so gloves and protective eyewear are non-negotiable. Inhalation of vapors can lead to dizziness or respiratory irritation, making good ventilation or fume extraction critical in workspaces. Spill containment measures and emergency showers must be accessible wherever the compound is stored or handled in bulk. The compound’s GHS classification and local regulatory status direct storage, transport, and disposal practices. Keeping up with safety data updates directly impacts worker health, incident response, and compliance during inspections.
Application Area
Acetophenone’s aromatic profile earns it a role in perfumes, soaps, and personal care products, blending easily with other fragrant compounds. In pharmaceuticals, the molecule becomes a stepping stone to medications for neurological and cardiovascular health or serves as an intermediate for anti-inflammatory drugs. Polymer and resin manufacturers sometimes use acetophenone as a cross-linking agent or solvent for specialty plastics. In research, its predictable reactivity grants scientists a flexible starting point for preparing custom molecules with new applications, whether for diagnostics, agricultural solutions, or advanced materials. Seeing acetophenone pop up across sectors underscores how this simple molecule underpins inventive technologies—even in spaces that don’t get much public attention.
Research & Development
Research around acetophenone continues to evolve. Teams have explored new catalytic pathways for its synthesis to cut waste and improve atom efficiency, including using recyclable solid acid catalysts or green oxidants that reduce environmental footprint. Some scientists keep pushing the boundaries, tweaking acetophenone’s structure to create precursors for newer pharmaceuticals or leading-edge polymer materials. Analytical chemists investigate how it behaves in complex mixtures, improving detection methods for both regulatory monitoring and forensic studies. The constant push for process intensification means every step—from raw material choice to waste minimization—gets scrutinized, driving advancements in both laboratory and plant settings. Investing in these research areas not only boosts profitability for producers, but also answers demands for safer and greener chemistry.
Toxicity Research
Toxicologists have investigated acetophenone’s effects for decades, examining acute and chronic exposure scenarios. In controlled animal studies, high doses affected the nervous system, but typical workplace contacts fall well below those thresholds. Regulatory agencies keep close tabs on both occupational limits and product safety, setting clear boundaries to protect workers and end consumers. Regular review of toxicology data sometimes leads to tighter standards and improved handling recommendations. What often goes unspoken is the importance of ongoing research—industry and independent labs both play key roles in generating new toxicity data as manufacturing methods evolve and as more complex uses for acetophenone emerge in new industries.
Future Prospects
Looking ahead, acetophenone’s story keeps growing. Trends in green chemistry drive researchers to craft cleaner, low-impact synthesis routes, possibly using renewable feedstocks or biocatalysis. Demand persists in established markets such as flavors and fragrance, yet newer applications appear in the design of smart polymers, agrochemicals, and diagnostic agents. Improvements in process safety, sustainability, and product purity can open up the doors to even wider uses. Expanding regulatory frameworks—driven by both health and environmental concerns—push producers and researchers to continually refine risk management, safe handling, and lifecycle assessments. With solid R&D investment and close attention to sustainability, acetophenone’s versatility promises even greater impact in decades ahead.
Everyday Encounters with Acetophenone
Step into a bakery, and the sweet vanilla aroma fills the air. The secret behind that familiar scent might surprise many people: acetophenone. Makers of fragrances and flavors use this compound because it brings a pleasant, almond-like note to perfumes and foods. As someone who loves the smell of a fresh pastry or a new bottle of cologne, it always fascinated me that a single molecule adds so much to products we use every day. Across my years of cooking and testing out new aftershaves, I started noticing how fragrance and flavor go hand-in-hand with chemistry—acetophenone, though colorless and oily on its own, has left a mark in daily life for well over a century.
Bigger Role in Pharmaceuticals and Solvents
Many people reach for over-the-counter medications when a headache strikes, not realizing the complex chain of chemicals and processes that made those pills possible. Acetophenone helps out in the background. Pharmaceutical labs use it to build molecules for medicines, making it a crucial intermediate for certain drugs. Chemists often choose acetophenone as a starting point because its structure easily links with other building blocks, paving the way for painkillers, antiseptics, and even some sleep aids. Years ago, I shadowed a pharmacist who remembered the old days of mixing compounds on-site, and acetophenone came up more than once as a useful “parent” molecule on the shelf.
Besides pills, acetophenone often ends up in solvents and resins. Paint and coatings depend on smooth solvents to keep liquids from clumping up or drying out too fast. Acetophenone helps with dissolving and blending, making paints easier to work with—something I learned quick as a novice DIYer frustrated by brush streaks and lumpy finishes on furniture. Professional painters turn to these types of solvents to avoid ruined jobs and wasted material.
Safety and Health Considerations
With all this utility, people wonder about safety. Acetophenone occurs naturally in some fruits and foods, but high levels can cause headaches, skin irritation, or dizziness. Production workers or lab technicians need gloves and good ventilation, especially over long shifts. Most people don’t come anywhere near concentrated acetophenone, but stories from plant workers remind us that chemicals have two sides—useful, yet demanding respect. The National Institute for Occupational Safety and Health (NIOSH) sets exposure limits to keep workplaces safe, and regular training goes a long way.
Sustainability and the Path Forward
Acetophenone usually comes from petroleum sources. Some researchers look for better ways to get it, maybe using renewable feedstocks or less energy-intensive methods. I’ve met chemistry students and professors who spend years piecing together green chemistry routes, aiming to cut down waste and emissions. Moving toward plant-based alternatives or closed-loop recycling could shrink the environmental footprint.
Reducing solvent use where possible also helps, and companies now push for more controls and alternatives. It goes to show that, even for a small molecule known by few outside the sciences, what we do with it—and how—matters more than ever. Getting safer, cleaner sources stands as the next big challenge, and many people already work to make it happen.
Understanding Acetophenone
Acetophenone pops up in more places than you might expect. It’s got this sweet, floral, almost almond-like smell, so folks have sprinkled it into perfumes for years. You’ll also stumble upon it in flavors or in the odd cough syrup because the scent masks other smells. Over time, chemical plants have relied on it as a building block for other products as well. But just because you catch a whiff of acetophenone in your everyday life doesn’t mean you can forget about the risks.
Looking at the Health Hazards
The science here is pretty solid. Acetophenone can irritate your eyes, skin, and respiratory tract, especially if you breathe it in at higher concentrations. Swallowing it in large amounts or breathing in concentrated vapors causes headaches, lethargy, or an upset stomach. You won’t get sick from catching the scent while strolling by a perfume counter, but if you work in a factory with the stuff, the stakes change.
According to the National Institute for Occupational Safety and Health (NIOSH), exposure limits exist for a reason. Folks working in plants don’t wear gloves and masks just for show. Extended contact—especially through the skin—may trigger rashes or blisters. There’s also some alertness around the nervous system. Animal studies show that really high doses can impact the liver or kidneys. The Occupational Safety and Health Administration (OSHA) pegs the safe airborne limit at 10 parts per million (ppm) for an eight-hour shift. That’s not a random number, but a protection based on known health data.
Environmental and Everyday Exposure
If someone dumps acetophenone into a river, fish and other creatures suffer too. Plants absorb it, and aquatic life reacts badly. It won’t stick around forever—it breaks down in sunlight—but for a while, it poses a problem. For most people, regular activities do not lead to high exposure. Using perfume or drinking soda flavored with traces of acetophenone does not push levels anywhere close to what’s seen in industrial accidents or unsafe workplaces.
Why It Still Matters
Every year brings stories of chemical leaks or unsafe conditions for workers. The Environmental Protection Agency (EPA) lists acetophenone as a substance worth monitoring. That’s not paranoia; it’s about keeping people safe at work and protecting water sources nearby. Not everyone checks safety labels or understands the difference between nontoxic and safe-at-low-levels. I’ve met plenty of folks on shop floors or labs who think, “If it smells sweet, it can’t be dangerous.” Chemical safety isn’t about fear—it’s about honesty.
Practical Solutions for Safer Use
Clear rules matter most. Factories need working hoods and workers need real gloves. Training beats warnings on a forgotten poster. For small-scale users, honest labeling and reference sheets at the point of sale keep people informed. On a policy level, enforcing routine air quality checks helps spot trouble before someone feels sick.
Schools and universities do their part too. Teaching the basics of chemical hazards gives people the real information—not rumors—so they understand when to voice a concern. Science isn’t about guesswork or fear, but about recognizing hazards and handling them with respect. Acetophenone isn’t as scary as some toxic heavy hitters, but nobody benefits by brushing off the facts.
Unlocking a Common Yet Overlooked Molecule
Walk into any college laboratory or look through the ingredients list of some fragrances, and acetophenone quietly claims its spot. Most folks never really think about the makeup of such chemicals, but for many in science, understanding these formulas can open up a clearer understanding of reactions, interactions, and real-world uses. Acetophenone shows up with the chemical formula C8H8O, which stands for eight carbon atoms, eight hydrogen atoms, and one oxygen atom. I found it surprising, during my own time working with organic chemistry, just how much can be packed into such a simple arrangement.
Why Knowing Chemical Formulas Matters
Staring at those letters and numbers, it’s easy to forget how much they tell us. Each group signals not only molecular size but influences how the chemical acts in the real world—things as simple as scent and as detailed as reactivity. That C8H8O formula isn’t random. The structure features a benzene ring (that’s six of those carbons) joined to a carbonyl group (a carbon-oxygen double bond), with a methyl group rounding out the rest—shaping everything from boiling point to the way chemists across industries decide to use it in new reactions. From experience, seeing how molecular structure influences a smell, or the way acetophenone dissolves in solvents, reminds me science has tangible effects outside the textbook.
Real-World Highlights and Safety Lessons
Products like flavorings, perfumes, and sometimes pharmaceuticals lean on meaty little molecules like acetophenone to get the job done. C8H8O makes appearances in plenty of places, and industrial labs use it to build more complicated compounds, or as a solvent. In my days around manufacturing, folks always asked about safety first. The chemical formula not only tells us what we mix into formulations, it guides those checks for toxicity, flammability, or reactions with other ingredients. Responsible handling and awareness of chemical properties stop accidents before they start.
Challenges and Solutions in Education
In high school and college classrooms, kids often glaze over during organic chemistry lessons. The theory alone turns intimidating, and I remember spending hours trying to visualize where each atom sat in that C8H8O puzzle. Visual models in the classroom—actual molecule kits, hands-on learning—help students turn abstract concepts into something they can grab onto. Digital tools and simulations let students see atoms snap together, making the pattern stick much more strongly than another page of written definitions.
Looking Ahead: Clear Information, Safer Choices
Decoding simple formulas like acetophenone’s means more than memorization. It’s about tracing the path from lab bench to product, making sure safety and performance line up every step of the way. Accurate and accessible information, grounded in real science, gives everyone from college students to workers on the production line the tools to engage confidently with chemicals that shape everyday life. The formula C8H8O turns out to have a bigger impact than meets the eye.
What You See and Smell
Acetophenone comes across as a colorless liquid, though with storage and exposure to light, a pale yellow tint can sneak in. Pour it out, and a strong, sweet scent that reminds some people of cherry blossoms or almonds fills the air. Chemists notice its similarity to bitter almond oil, and it shows up in plenty of fragrance formulations. For folks working in a lab, its smell quickly becomes familiar—telltale, almost comforting—but also a hint to use proper ventilation. Working with it day after day, I've found it can hang in the air, so quality exhaust fans make a big difference.
Feeling Its Weight and Measuring the Boil
With a molecular weight of around 120 grams per mole, acetophenone flows easily, neither syrupy nor watery. People in the lab often check its boiling point: about 202°C or 396°F. That makes it less volatile than some organic solvents, but it still evaporates if left open. I've learned not to leave flasks uncovered, as missing sample can mean lost time or ruined results. Pouring it on a chilly day, you’ll spot it thickening up—its melting point lands right at 19-20°C, which sits close to a cool indoor room. In winter, bottles left on a cold shelf might develop crystals. Gentle warming brings it back to liquid, but the switch is a real concern for storage and transport.
Mixing In and Dissolving Out
Solubility tests tell plenty about a chemical, especially in a research environment. Acetophenone blends smoothly with most organic solvents, like ethanol, ether, and benzene. It hardly mixes with water, though—expect only a few grams to mix in a whole liter. This trait limits how labs handle spills and cleanup; spill kits for organic solvents are essential. Acetophenone's density hovers around 1.03 grams per cubic centimeter. Pour it in a separatory funnel with water, and you’ll notice it forming a layer just underneath, which makes extractions straightforward but also demands careful separation.
Light, Storage, and the Safety Angle
People who store acetophenone should watch out for light exposure. It tends to change color over time—it may take on a yellow hue after a stint in sunlight or under strong laboratory lights. This change can mean impurities are forming, which sometimes influences results in sensitive tests. From my own work, I’ve moved all acetophenone stocks into amber glass bottles and kept them off the lab benches. Temperature swings matter too, not just for safety but to avoid freezing or unexpected solidification.
Why the Physical Side Matters
If you’ve ever handled large batches, you know packaging matters. Glass bottles work well but must be kept from the edge of benches—acetophenone eats through some plastics, so switching containers mid-work can cause real trouble. For anyone blending flavors, fragrances, or pharmaceutical agents, the boiling and melting points spell out exact handling needs—nobody wants a solvent drying out mid-mix or crystallizing in storage. High school chemistry books mention these points, but in the real world, these properties hit home: wasted materials, failed experiments, and safety risks. Real experience—and a few mistakes—make these physical properties something you remember, not just memorize.
Keeping Labs and People Safe
So how can the risks be handled? Clear labeling helps. I’ve learned the value of double-checking bottles and dating solutions. Safety showers and eyewash stations must stay working, since splashes and vapors—though less irritating than some solvents—still cause headaches and eye stings. Good training for young researchers, vigilance for older hands, and plenty of backup stock in appropriate containers keep work safe and productive.
Common Sense Meets Chemistry
Acetophenone shows up in labs across the world, finding its way into everything from perfumes to complex chemical syntheses. This liquid carries a sweet scent, almost like almonds or cherries, but behind the pleasant aroma, it brings risks that shouldn’t be ignored. The way this substance gets stored and handled plays a big role in keeping people safe and operations smooth.
Forget Stashing It Anywhere: Storage Demands Real Care
Anyone who’s worked near organic solvents recognizes the smell—sharp, persistent, and a telltale sign to check the label. Acetophenone sets off more than just olfactory triggers. Its flash point sits low, making it more flammable than some folks realize. Putting it on a shelf near open flames, heaters, or direct sunlight ramps up the danger fast.
Always keep acetophenone in a tightly closed container. Glass works best since plastics sometimes react or get soft with chemicals like this. Store containers in a cool, dry, and well-ventilated area, far from incompatible substances such as strong oxidizers, acids, and bases. Locking up chemical shelves and keeping a good inventory list puts another barrier between a curious visitor or distracted worker and a possible spill.
Eyes, Lungs, and Hands—Protect Them All
Back in my early lab days, a quick splash from a pipette woke me up to acetophenone’s sting—red eyes and a scratchy throat that lingered for hours. Always suit up. Safety goggles, chemical-resistant gloves (nitrile or neoprene beats old-school latex, which lets solvents slip through), and a lab coat prevent most mishaps. Good ventilation means a lot: use a fume hood when transferring or measuring out acetophenone. Inhalation pulls vapors deep into your lungs, and a headache or nausea might hit before you realize what happened.
Spill Happens: Stay Ready
The best labs don’t rely on luck; they rely on planning. Any time a bottle slips or something tips, absorb the spill using sand or a commercial spill kit. Never let acetophenone drain away into sinks or outside. After cleaning, bag up contaminated materials, label the waste, and arrange for hazardous waste collection. Letting these details slide may bring not just workplace danger, but legal headaches as well.
Label Everything and Train Everyone
People get careless in familiar settings and forget the dangers that chemicals carry. Clear labeling—hazard signs and instructions—reduces mistakes. Training should be regular, hands-on, not just a dull slide show. I once watched an untrained team member reach for acetophenone to “clean up” a gluey workbench; a friend stopped him just in time. Explaining why safety matters sticks better than rules alone.
Cut the Risk Short: Choose Safer Substances When Possible
Sometimes, a safer substitute can do the same job and lower risk. For small-scale or teaching labs, look for alternatives if the procedure allows. If acetophenone remains necessary, never cut corners to save time or money. Fires, chemical burns, or long-term health problems cost more than any shortcut ever saved.
Storing and handling acetophenone isn’t just a checklist; it’s a culture. Small habits—tight lids, clean workspaces, regular checks—build layers of protection. Doing it right keeps everyone healthier and workplaces running without disaster. Experience taught me it’s the daily choices, not just big policies, that make the difference.
| Names | |
| Preferred IUPAC name | 1-Phenylethan-1-one |
| Pronunciation | /əˌsiːtəˈfəʊnəʊn/ |
| Identifiers | |
| CAS Number | 98-86-2 |
| Beilstein Reference | 635848 |
| ChEBI | CHEBI:27879 |
| ChEMBL | CHEMBL14052 |
| ChemSpider | 564 |
| DrugBank | DB02542 |
| ECHA InfoCard | 100.62.9 |
| EC Number | 200-851-8 |
| Gmelin Reference | 715 |
| KEGG | C01341 |
| MeSH | D000077298 |
| PubChem CID | 7410 |
| RTECS number | AL3150000 |
| UNII | LT7FXWFDI6 |
| UN number | UN1990 |
| Properties | |
| Chemical formula | C8H8O |
| Molar mass | 120.15 g/mol |
| Appearance | Colorless liquid |
| Odor | Sweet, pungent, almond-like |
| Density | 1.028 g/mL at 25 °C |
| Solubility in water | Moderately soluble |
| log P | 1.65 |
| Vapor pressure | 0.4 mmHg (20°C) |
| Acidity (pKa) | pKa = 20 |
| Basicity (pKb) | 14.75 |
| Magnetic susceptibility (χ) | -41.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.533 |
| Viscosity | 1.029 cP (20°C) |
| Dipole moment | 3.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 199.0 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −49.7 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3096.7 kJ/mol |
| Pharmacology | |
| ATC code | N05CH06 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 2, Instability: 0, Special: - |
| Flash point | 77 °C |
| Autoignition temperature | 615 °C (1139 °F; 888 K) |
| Explosive limits | Explosive limits: 2 – 11% |
| Lethal dose or concentration | LD50 oral rat 815 mg/kg |
| LD50 (median dose) | LD50 (median dose): 815 mg/kg (oral, rat) |
| NIOSH | PH1575000 |
| IDLH (Immediate danger) | 300 ppm |

