Aniline: A Journey Through Science, Industry, and Possibility
Historical Development
Aniline has a story that threads across two centuries. In 1826, Otto Unverdorben distilled indigo to produce "crystallin," later known as aniline. August Wilhelm von Hofmann mapped out its structure and methods of preparation by the 1840s. Among the biggest turning points came in 1856, when William Henry Perkin created mauveine, the first synthetic dye, using aniline. The vibrant purple hue sparked the synthetic dye industry and made aniline a household name in chemistry. Chemists after Perkin pushed aniline into new territory, creating a wide palette of dyes rivaling anything nature could provide. It marked the beginning of using coal tar as a resource, bringing Germany center stage in chemical manufacturing. Over time, the discovery shaped not only the industry surrounding color, but the pharmaceutical and plastics world as well.
Product Overview
Aniline, or phenylamine, stands out as a primary aromatic amine. With the formula C6H5NH2, it serves as a starting point for a range of chemical products. Modern aniline features in polyurethanes, dyes, rubber processing, herbicides, and pharmaceuticals. Its tie to the iconic "aniline dyes" industry persists, but today's synthetic plastics sector relies heavily on it. In terms of raw material, aniline often comes from the reduction of nitrobenzene, drawn from benzene, which connects it closely to petrochemicals. In the global context, China leads production, followed by the United States and Europe, reflecting changing patterns in industrial capacity.
Physical & Chemical Properties
Aniline appears as a colorless to slightly yellow liquid in pure form. Aging or exposure to air can turn it brown due to oxidation. At room temperature, it exudes a distinct smell reminiscent of rotten fish. Its boiling point sits around 184°C, with a melting point slightly below zero Celsius. Aniline dissolves sparingly in water but mixes well with most organic solvents, like ethanol and ether, because of its polar nature. The amine group (–NH2) sticks out on a benzene ring, giving it both nucleophilic and basic character. Aniline reacts relatively easily compared to benzene, allowing chemists to tailor it through acetylation, sulfonation, and alkylation. Its refractive index and moderate vapor pressure mean it doesn’t evaporate rapidly under normal conditions. Aniline burns with a smoky flame, hinting at incomplete combustion and formation of toxic byproducts if handled carelessly.
Technical Specifications & Labeling
Every shipment of aniline gets tracked through rigorous documentation. The chemical industry leans on standards, like those set by ASTM and ISO, to define purity above 99.5% for most applications. Common impurities include water, phenol, and nitrobenzene. Manufacturers provide batch analysis with each lot, outlining physical and chemical data—boiling point, refractive index, density, water content, and GC purity by area percent. Labels include hazard symbols—indicating toxicity, flammability, and potential for environmental harm. The UN number for aniline is 1547, marking it for regulated transport. Safety Data Sheets (SDS) follow the GHS approach, ensuring response protocols for spills, burns, and inhalation are well-outlined. Packaging spans steel drums, bulk ISO tanks, and specialized containers lined to prevent corrosion and minimize vapor loss.
Preparation Method
In the industrial world, producing aniline starts with benzene. The nitration of benzene produces nitrobenzene, typically using mixed acid (a blend of concentrated sulfuric and nitric acids). This step is exothermic and demands careful heat management. Next, nitrobenzene gets reduced to aniline through catalytic hydrogenation, often with a palladium, nickel, or copper catalyst, under pressure and temperature control. Another established route uses iron filings and hydrochloric acid, a method rooted in 19th-century laboratory chemistry. Synthetic pathways remain tightly controlled because byproducts, such as azobenzene and phenol, can reduce yield or introduce impurities. Advances in catalyst technology have improved selectivity and efficiency, lowering energy use and minimizing waste streams.
Chemical Reactions & Modifications
Aniline’s structure opens up countless synthetic possibilities. Its amino group activates the benzene ring toward electrophilic substitution, allowing reactions like bromination, chlorination, and sulfonation to proceed under milder conditions than benzene itself. Room temperature bromine gives tribromoaniline instantly, highlighting its unusual reactivity. Aniline forms diazonium salts with nitrous acid, which unlock further chemistry—chief among them azo coupling to create dyes and pigments. Its acylation with acid chlorides or anhydrides gives acetanilide and related derivatives. Through alkylation, sulfa drugs and analgesics spring from this simple amine. Its role as a nucleophile also places it at the heart of urethane, rubber antioxidant, and pesticide manufacturing. Transformation into methylene diphenyl diisocyanate (MDI), for polyurethanes, remains one of the cornerstone uses.
Synonyms & Product Names
Aniline answers to many names in labs and factories: phenylamine, aminobenzene, benzenamine, and even amidobenzene. In dye trade and pharmaceuticals, people sometimes see it called "oil of mirbane," though this originates from nitrobenzene. Some older labels reference derived products, like blue oil or aniline oil, dating back to the color chemistry boom. Product codes and CAS numbers—mainly 62-53-3—standardize identification in registries and compliance paperwork to avoid confusion between suppliers and between nations.
Safety & Operational Standards
Handling aniline never calls for casual work. Its toxicity can sneak in through inhalation, skin contact, and ingestion. Workers use chemical-resistant gloves, splash-proof goggles, and, in many places, full-face respirators. Chronic exposure leads to methaemoglobinaemia, turning blood less able to carry oxygen and causing headaches or cyanosis in affected workers. Short-term symptoms include dizziness, nausea, and skin irritation. Strict maximum allowable concentrations, often below 2 ppm in air, drive engineering controls—exhaust ventilation, fume hoods, and leak detection sensors. Fire authorities list aniline as a flammable liquid. Fire suppression uses foam, dry chemical powder, or carbon dioxide; water jets spread it or trigger decomposition to hazardous gases. Internally, companies enforce regular medical checkups for workers and stringent environmental monitoring to address accidental releases. Modern standards expect every storage tank and process line to feature secondary containment, double-walled piping, and sensor alarms for rapid shutdown. Regulators check waste streams for aniline, especially as contaminated sites from decades past still create headaches in cleanup and liability.
Application Area
Aniline’s role as a chemical intermediate far outpaces its direct consumer use. Polyurethane plastics—essential for insulation, furniture, automobiles, and adhesives—draw on the MDI produced from aniline. Pesticides like carbaryl and pharmaceutical agents for fever or pain relief start with simple aniline chemistry. In the textile sector, aniline dyes still find use in specialty and heritage products, though environmental controls constrain their widespread application. Rubber chemicals, such as antioxidants, keep tires and belts in top shape for longer stretches. Engineers in water treatment sometimes dose aniline derivatives as corrosion inhibitors. Research labs count it as a key compound for synthesizing more elaborate molecules, especially for academic or medicinal discovery.
Research & Development
R&D divisions still chase better routes for preparing aniline and its derivatives, both to shave production costs and reduce waste. Catalysis research races forward, with new metal systems demonstrating greater selectivity or working under milder conditions. Biomass-based benzene and renewable hydrogen efforts give hope for greener production, bypassing the fossil fuel chain altogether. Advanced computer modeling helps chemists design aniline analogues targeted for pharmaceuticals: antihistamines, anticancer agents, and antibiotics often begin on a lab bench with aniline scaffolds. In polymer science, tweaking aniline’s reactivity yields new coatings and conductive polymers. Environmental scientists analyze how to keep aniline out of waterways, improving efficiency of biofilters and advanced oxidation treatments.
Toxicity Research
Research on the hazards of aniline signals many challenges. The substance causes methaemoglobinaemia, even at low exposures, which proves particularly dangerous for infants and those with certain enzyme deficiencies. Studies spanning decades note carcinogenic potential, though epidemiological data in humans remains mixed. In animal trials, high doses trigger tumors and organ damage. Regulatory bodies like OSHA and the EU place strict upper limits on workplace and environmental levels. Wastewater from aniline production receives close scrutiny; technology for detecting parts-per-billion concentrations now forms part of routine site monitoring. Medical training focuses on rapid diagnosis and treatment—methylene blue counteracts methaemoglobinaemia, and early intervention prevents most long-term health problems. Safety research also explores less hazardous aniline derivatives, hoping to keep industrial utility without the historical baggage of toxicity.
Future Prospects
Tomorrow’s aniline industry faces both promise and scrutiny. Sustainability concerns drive inquiries into bio-based benzene, renewable hydrogen, and fully closed-loop production. New catalysts can cut energy use and minimize byproducts, squaring efficiency with environmental demands. As markets look for more biodegradable plastics, modifications in aniline chemistry could set new standards in polyurethane and resin performance. Green chemistry protocols, beyond simply reducing emissions, now push manufacturers to track every atom, aiming to reclaim or neutralize all byproducts. Medical chemists eye aniline analogues for next-generation drugs, thanks to their binding flexibility and synthetic versatility. Industry competitors in China, India, and Southeast Asia rise with new capacity, reshaping trade flows and intensifying focus on compliance and transparency. Demand for insulation and specialty chemicals keeps growth prospects strong, yet regulatory developments around worker safety and product stewardship remain in constant motion.
The Building Block Behind So Many Everyday Goods
Aniline doesn’t get a flashy spot in most conversations. Still, this stuff weaves through so much of what's around us—inside rubber soles, color in your jeans, the foam in your car seat. The reason? It opens doors for chemists to create all sorts of important products.
Take a look under your feet. If you have sneakers with cushy soles, chances are, aniline’s in them. Those foams that make life more comfortable—like what lines office chairs or keeps insulation tight in the walls—come from polyurethane, which starts with aniline. Polyurethane foam couldn’t exist in the form we know it without this chemical. Back in my college days in a chemistry lab, I remember the heavy, sweet smell coming off aniline as we made small test batches of polyurethane. Even the smallest spill stuck around as an odor for days; one drop left in a beaker was enough to remind you just how much punch this compound packs.
Aniline Adds Color to Life
Dyes and pigments owe a lot to aniline. The first synthetic dye, mauve, sprang from this chemical in the 1800s. These days, textile factories rely on aniline for vivid blues—think denim blue in your favorite pair of jeans. It’s why just about every corner store sells clothing with colors that last through wash after wash. When I worked in a textile mill one summer, I learned how synthetic and stable colors changed the game. Old methods always ran or faded; not so much since aniline dyes took over.
Medicine and Everyday Cleaners
Pharmaceuticals also draw from aniline's chemical structure. Painkillers like acetaminophen trace their origins to it. Drug manufacturers use aniline as a starting point for many therapies, from fever reducers to treatments for some psychiatric conditions. Even today, while shopping for cold medicine, I think of how this compound made it from factory floors to medicine cabinets.
On the cleaning side, a lot of industrial chemicals used as disinfectants or germicides also started out as aniline derivatives. They might not be the headline ingredient in your home spray bottle, but they keep hospital surfaces and surgical equipment clean, which makes a difference for patient safety.
Concerns and Safer Use
Aniline isn’t perfect. In large doses, it can be toxic. Factory workers handling it need solid safety training and good equipment—respirators, gloves, and strict rules on ventilation. Regulatory groups like OSHA set limits in workplaces to help avoid unnecessary risks. Stories float around in every chemical plant about someone who underestimated aniline and ended up with blue fingers—methemoglobinemia can set in fast. Community and industry need open conversations about these risks, regular inspections, and clear labeling.
Looking Ahead
We keep searching for better ways to use and substitute for aniline when it makes sense, especially in sensitive environments. Green chemistry is pushing for plant-based alternatives, and researchers are finding clever routes to cut waste. With more attention to safety and impact, aniline continues to help shape modern life, even if most folks never realize it.
Understanding Aniline’s Hazards
Factories and labs have used aniline for over a century. This chemical helps make dyes, plastics, and drugs. Folks working in these settings often come face to face with aniline, or at least breathe the air where it lingers. Yet, many outside of science circles barely know the name, unless they’ve scanned a safety sheet or read a label.
Getting straight to the point — aniline earned a spot on lists of hazardous chemicals for good reason. Breathing in its fumes, or letting the liquid touch skin, can have serious effects. Studies published by the Centers for Disease Control and Prevention (CDC) show aniline can pass through the skin and reach the bloodstream. Red blood cells carry oxygen, but aniline interferes with this job. The medical term methemoglobinemia is a mouthful, but its symptoms — blue lips, headaches, confusion — aren’t hard to spot. In bad cases, someone could have trouble breathing or collapse, especially if they aren’t treated right away.
Risks at Work and for the Environment
Many people think risk is limited to busy chemical plants. That’s true for direct exposure, but accidents can send aniline outside. Spills and leaks have polluted rivers and soil before, harming fish and wildlife. The US Environmental Protection Agency (EPA) keeps a close eye on aniline as a hazardous pollutant, and cleanup crews treat it seriously during a spill.
Personal stories can be more revealing than statistics. I remember a friend working in a dye factory who developed rashes on his hands after a splash. He shrugged it off at first. After more headaches and feeling winded, he realized aniline wasn’t something to take lightly. He started using better gloves and made sure the ventilation system actually worked. It made a difference.
Why It Matters to Everyday People
Almost nobody outside a plant would come across pure aniline at home. Still, that doesn’t mean the issue can be ignored. Finished goods, like rubber products and dyes, might leave behind traces. Researchers found aniline in cigarette smoke, and trace amounts in hair coloring products. International agencies, such as the World Health Organization, point out that these small amounts rarely cause harm for regular folks — the dose makes the poison.
Taking Sensible Steps
Clear guidelines exist for handling and storage. Protective gear matters. Gloves and goggles save skin and eyes, while proper containers stop fumes from seeping out. Good ventilation cuts down accidents. Supervisors and safety coordinators have a responsibility to train workers and check equipment. Regular training and honest reporting keep disasters at bay.
Talking openly about chemicals like aniline helps ground the conversation in reality. Public agencies, labs, and workers need to keep pushing for stricter checks and share findings with each other. If you work where aniline is used, there’s no sense taking unnecessary risks. Know your facts, demand good equipment, and speak up if something feels wrong. Small steps add up, and they keep communities and families safe.
Understanding the Risks
Aniline pops up in many industries. From dyes and rubber manufacturing to pharmaceuticals, it’s a chemical plenty of folks come across. Still, just because it’s common doesn’t make it harmless. My first brush with aniline came in a small production plant. I watched a co-worker handle open containers without gloves, and hours later, complaints started rolling in—nausea, dizziness, confusion. Safety briefings make a real difference.
Direct Exposure: Don’t Take Chances
Aniline absorbs into the body through skin and lungs. Even low doses can affect oxygen levels in blood—methemoglobinemia isn’t just a mouthful, it’s a serious risk. Breathing in vapors can leave you light-headed or short of breath. Contact with skin can lead to irritation and even long-term health problems.
The scientific data rings clear: NIOSH states that exposure must stay under 2 parts per million over eight hours. Short spikes above that raise red flags. Given its toxicity, good habits keep exposure in check.
Protective Gear Saves Lives
Companies can’t afford shortcuts here. Whenever handling aniline, proper gear matters. Nitrile, butyl, or neoprene gloves do the trick—bare hands won’t cut it. Chemical splash goggles and face shields shield eyes from stray droplets. A simple cotton mask doesn’t block vapors, so at-risk workers turn to organic vapor respirators. Changing out filters regularly stops them turning into a hazard themselves.
Full coveralls or aprons made from chemical-resistant material set a boundary between your skin and the substance. If aniline touches clothing, get changed fast. Wash any contaminated skin with warm water and soap, not just a quick rinse.
Ventilation and Spills
From experience, good ventilation never lets fumes sneak up. Fans and fume hoods put a constant barrier between workers and hazardous vapors. Relying on open windows or AC vents isn’t enough. Every shop needs spill kits close by—absorbent pads, neutralizers, and disposal bags. Raw rags or a mop won’t do the job safely.
If a spill happens, alert the team, leave the area if the air grows strong with chemical smell, and let trained staff manage the cleanup. For small puddles, barrier socks contain the liquid; for larger spills, evacuate non-essential workers and grab professional help. Keep fire extinguishers on hand. Aniline vapors don’t need much of a spark to ignite.
Storage and Waste Management
Storing aniline calls for cool, well-ventilated spots away from direct sunlight. Containers stay tightly sealed and labeled with chemical hazard symbols—no room for guessing. Don’t keep the chemical near acids, oxidizers, or food storage. In my last factory, one careless stacking move mixed incompatible chemicals, leading to a dangerous release. It only takes one slip-up.
Disposing of aniline runs through proper channels. Never dump it down a drain or regular trash. Licensed hazardous waste handlers should transport and process it. Regular workers shouldn’t take on this job; mistakes risk community safety.
The Human Factor
Complacency lets accidents creep in. Regular training and drills keep safety fresh in everyone’s mind. Encourage teams to speak up about leaks, missing labels, or faulty gear. No one should work with aniline alone—emergencies come fast, and having another set of eyes means faster help if something goes wrong. Earning trust through clear rules and open communication sharpens everyone’s awareness. That’s what keeps the workplace safe, shift after shift.
Chemical Identity: What Makes Aniline Stand Out
Aniline carries the chemical formula C₆H₅NH₂. This means a benzene ring—the backbone for plenty of molecules—hooks up with an amino group, NH₂. In less technical terms, aniline’s structure looks like six carbon atoms in a hexagonal ring, attached to a single group containing nitrogen and hydrogen. To someone who’s cracked open an organic chemistry textbook, this is a classic starter molecule, often drawn out during lessons for its straightforward combination of aromatic and amine features.
Why Aniline Matters Beyond Textbooks
Aniline feels like a quiet force in industry. You won’t see its name splashed in headlines, yet it shapes huge aspects of daily life. Take dyes: synthetic colors in fabrics often trace back to aniline as a critical ingredient. Without this compound, our options for vibrant clothing would shrink. The same goes for some medicines and rubber products—each relies on aniline chemistry to function at its best. Factory floors that create rubber for tires or pharmaceutical plants with big-name pain relievers would hit a roadblock without it.
Personally, my respect for aniline grew during an internship at a dye-manufacturing plant. Scientists there discussed aniline as often as you’d talk about flour in a bakery: totally essential, rarely dramatic, but always present. They’d measure purity and debate safer handling. I saw how one molecule could shape jobs, profits, and even consumer trends through subtle chemical tweaks.
Handling Risks: It’s Not All Smooth Sailing
With all its utility, aniline brings real safety issues. Breathing in its fumes or letting it touch skin raises health hazards—headaches, dizziness, rashes, and worse if exposure runs high. Factories spend big money on tight safety protocols and monitoring, since a slip-up could mean hospital visits for workers or fines for the company. Some of my old colleagues recounted stories of chemical leaks—quick action and protective gear kept trouble small, but each incident stressed the need for solid training and robust emergency plans.
The world produces millions of tons of aniline every year. Transporting and storing this chemical takes planning, and regulations shape almost every step. For example, in the United States, OSHA (Occupational Safety and Health Administration) and EPA (Environmental Protection Agency) set out rules on exposure levels and waste disposal. Strong oversight helps limit spills or contamination, but real safety starts with people on the ground, not just paperwork in a drawer. My time in industrial plants showed me that even the best rulebook falls flat if operators ignore the details.
Better Solutions: Aiming for Green Chemistry
Some companies look for greener ways to make and use aniline, hoping to cut pollution and protect health. Catalysts with fewer side effects have started showing up in pilot projects. Cleaner solvents and tighter recycling loops offer hope. The hurdles aren’t just technical—costs and old habits slow adoption. But inside labs, researchers keep pressing. The rewards stretch from improved worker safety to less impact on rivers and soil.
The push for safer chemicals matters in every corner. As public awareness about industrial chemicals rises, companies willing to invest in better processes and technologies stand a good chance of leading the pack. Aniline’s story highlights how conscious choices, careful engineering, and continued investment can change an industry for the better.
Aniline Isn’t Just Another Chemical
Aniline turns up everywhere—from making dyes and rubber to pharmaceuticals. Left alone on a shelf, it doesn’t seem too wild, but the risks climb quickly when someone ignores basic safety. At room temperature, it sends off vapors that belong nowhere near your lungs and can seep through skin. I’ve learned in the lab that a shortcut or a cracked seal turns into more trouble than it’s worth.
Getting Storage Right Saves Headaches and Health
A dusty bottle in a forgotten corner, exposed to light and heat, can form toxic byproducts or even catch fire. So, using airtight containers built from materials that aniline can’t eat through isn’t just smart, it’s non-negotiable. Steel drums lined with epoxy or glass bottles with sealed caps stand up to the job. Store it away from sunlight and don’t stack heavy containers on top, since pressure can warp seals. Temperature controls matter too—a dry, cool room with forced ventilation gives everybody peace of mind.
Don’t store aniline near acids or oxidizing agents. Mixups create dangerous situations far faster than most expect. I’ve seen chemical storerooms where color-coded containers and clear labeling stop disasters before they start. Employees get regular training, not just a pamphlet once a year. These habits prevent confusion and injuries.
Don’t Cut Corners on Personal Protection
Splash goggles, nitrile gloves, thick aprons, and sometimes even a full-face respirator—these aren’t overkill. Since aniline causes skin absorption hazards, every layer drops risk. It only took one coworker skipping gloves for me to see why. Proper engineering controls, like local ventilators above workstations, pull away vapors before they drift too far.
Disposing of Aniline Responsibly
Dumping aniline down a drain or tossing it into regular trash should never cross anyone’s mind. Even small spills leach into groundwater and poison fish, plants, and people. Most areas treat aniline as hazardous waste, so collection happens in chemical-resistant drums or carboys labeled with a hazard sticker and log sheet. Local environmental agencies handle the rest, often through incineration at licensed facilities.
At my old workplace, we logged every outgoing container with weight, date, and chemical type. If anything looked off, inspections followed. That record-keeping builds a trail for regulators and takes the guesswork out of tracking the chemical’s journey. For small users, community hazardous waste events offer the safest way to hand off leftovers. Industrial settings contract professional waste haulers, who never accept unlabeled vessels.
Education and Consistency Build Trust
The worst policies only make sense on paper. Regular drills, honest audits, and a culture where anyone can call out unsafe storage mean everything. I remember a plant’s manager putting their own emergency numbers on chemical cabinets. That simple act raised everyone’s stakes and made safety personal. Schools and workshops can’t ignore these lessons if they want science to serve, not harm, communities.
Following clear rules around aniline isn’t burdensome so much as responsible. Storing and disposing of it with vigilance preserves human health and keeps the environment out of harm’s way.
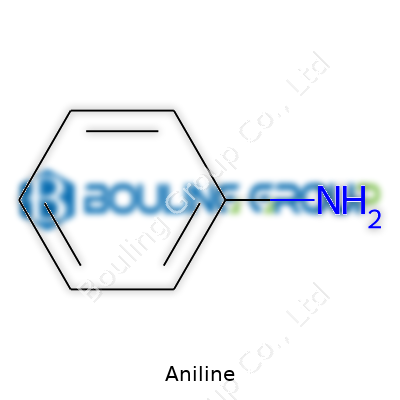
| Names | |
| Preferred IUPAC name | Benzenamine |
| Other names |
Phenylamine
Anilin Aminobenzene Benzenamine |
| Pronunciation | /ˈæn.ɪ.liːn/ |
| Identifiers | |
| CAS Number | 62-53-3 |
| Beilstein Reference | 605293 |
| ChEBI | CHEBI:17296 |
| ChEMBL | CHEMBL123 |
| ChemSpider | 542 |
| DrugBank | DB01892 |
| ECHA InfoCard | 100.002.700 |
| EC Number | EC 200-539-3 |
| Gmelin Reference | 381 |
| KEGG | C01444 |
| MeSH | D000886 |
| PubChem CID | 6115 |
| RTECS number | BX1925000 |
| UNII | 10M314UL39 |
| UN number | 1547 |
| Properties | |
| Chemical formula | C6H7N |
| Molar mass | 93.13 g/mol |
| Appearance | Clear, oily, colorless to slightly yellow liquid |
| Odor | Aromatic amine-like |
| Density | 1.0217 g/cm³ |
| Solubility in water | 3.6 g/100 mL (20 °C) |
| log P | 0.90 |
| Vapor pressure | 0.6 mmHg (20°C) |
| Acidity (pKa) | 4.6 |
| Basicity (pKb) | 9.4 |
| Magnetic susceptibility (χ) | -6.8×10⁻⁹ cm³/mol |
| Refractive index (nD) | 1.586 |
| Viscosity | 3.86 cP (20°C) |
| Dipole moment | 1.50 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 87.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 86.6 kJ mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3267 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D01AE05 |
| Hazards | |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled; causes damage to organs; suspected of causing cancer; harmful to aquatic life. |
| GHS labelling | GHS02, GHS06, GHS07 |
| Pictograms | GHS02,GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | Harmful if swallowed. Toxic in contact with skin. Toxic if inhaled. Causes skin irritation. Causes serious eye irritation. Suspected of causing genetic defects. Suspected of causing cancer. Causes damage to organs through prolonged or repeated exposure. |
| Precautionary statements | P261, P264, P270, P271, P301+P310, P302+P352, P304+P340, P308+P311, P312, P330, P363, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-2-Acidity |
| Autoignition temperature | 630 °F (332 °C) |
| Explosive limits | 4.2%–19% |
| Lethal dose or concentration | LD50 oral rat 250 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Aniline: 850 mg/kg (oral, rat) |
| NIOSH | NIOSH: BZ6125000 |
| PEL (Permissible) | 2 ppm |
| REL (Recommended) | REL-TWA 0.1 ppm (0.44 mg/m3) |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Acetanilide
Azobenzene Benzenediazonium chloride Cyclohexylamine Diphenylamine Phenol Phenylhydrazine Phenylenediamine Sulfanilamide Toluidine |

