Diisobutyl Adipate: A Deep Dive into Its Journey and Future
Historical Development
Diisobutyl Adipate, known in technical circles as DIBA, traces its roots to the early expansion of plasticizer chemistry during the twentieth century. As industries sought new ways to make plastics flexible and resilient, the search for reliable esters centered on the transformation of adipic acid with different alcohols. DIBA, branched and less volatile than many cousins, gained traction among manufacturers who wanted non-greasy, mild solutions. By the 1960s, its value in cosmetic and polymer fields was set, driven mostly by a practical demand for plasticizers and emollients that blended in easily, left no lingering odors, and didn’t irritate the skin. Synthetic routes improved, crude mixtures gave way to purer grades, and regulatory frameworks evolved to manage emerging data about exposure and toxicity.
Product Overview
DIBA has carved a solid place in the portfolios of chemical suppliers. It’s mainly established as a specialty ester that looks like a clear, slightly oily liquid, easy to pour, with no sharp scent—a feature that people handling it in factories or labs definitely appreciate. Many companies choose DIBA as much for what it doesn’t do—leave sticky residues, yellow with age, or corrode delicate surfaces—as what it adds to a product. Blending properties allow it to combine efficiently with cosmetic formulations, plastic compounds, and even inks. Offered under brands like Plastomoll I, EINECS 203-090-1, or just Diisobutyl Hexanedioate, it travels to market with a short ingredient list, clear labeling, and usually arrives in steel drums or plastic totes for direct transfer to blending tanks.
Physical & Chemical Properties
Diisobutyl Adipate has a molecular formula of C14H26O4 and a weight just over 258 g/mol. You’ll see it pour as a colorless to pale-yellow liquid. It barely dissolves in water but mixes well with most organic solvents and key resins. It has a boiling point beyond 320°C and flashes at about 170°C; those values matter more than ever as firms tighten safety protocols for high-heat processes. The density hovers around 0.95 g/mL at room temperature. Its low viscosity helps labs and production lines avoid clumping; it blends in gently with other liquids, reducing problems with mixing machinery. Chemists appreciate its stability under moderate temperatures. Chemical engineers exploit its resistance to hydrolysis over most storage conditions, giving it a shelf life of several years if the surroundings stay dry and cool.
Technical Specifications & Labeling
Industrial customers demand detailed technical data, and DIBA delivers. Most suppliers guarantee a purity above 99 percent, trace moisture content under 0.1 percent, acid value under 0.1 mg KOH/g, and color index below 30 APHA. Chemical Safety Data Sheets highlight its CAS number, 141-04-8, along with European REACH registration and labeling codes. Labeling always notes the substance’s flammability risk and standard PPE requirements—protective gloves, goggles, proper ventilation. On batch certificates, you’ll find full traceability back to lot and manufacture date. Those who’ve seen regulatory audits know the headaches that missing labels and incomplete logs can bring, so consistent markings and up-to-date documentation prevent regulatory bottlenecks later.
Preparation Method
Producing DIBA starts with reaction between purified adipic acid and excess isobutanol in the presence of acid catalysts. Typical plants use continuous stirred reactors, heat to about 140°C, and recover water that forms as a byproduct. The process produces a crude ester, which then undergoes multi-step purification—often vacuum distillation—before being sent for quality checks. Some companies opt for solid catalysts to reduce neutralization waste, an approach that’s gaining ground thanks to stricter waste management rules. As reactors churn day and night, quality teams regularly sample intermediates to control byproduct formation and minimize unreacted alcohol. Chemical engineers constantly push for higher yield with fewer side products, since disposal adds hidden costs and lowers overall sustainability.
Chemical Reactions & Modifications
DIBA’s core structure—adipic acid’s six-carbon backbone plus two branched isobutyl chains—offers flexibility for further chemical reactions. While mild and long-lasting as a stand-alone plasticizer, researchers sometimes tweak its backbone through transesterification to develop new polymer compatibilities. Niche sectors, like specialty coatings and lubricants, test mild modifications to see if extra groups change its wetting or softening behavior. Large-scale chemical companies, always watching for patents, know modifying DIBA opens doors to custom blends for medical or aerospace use. These modifications rarely enter the consumer domain but build crucial specialty value for suppliers wanting something competitors can’t copy easily.
Synonyms & Product Names
DIBA appears in international commerce under a stack of alternative names. Diisobutyl Hexanedioate, Adipic Acid diisobutyl ester, and EINECS 205-090-1 all refer to the same base product. Regulatory harmonization means most containers ship with multiple synonyms on the barcode label, easing customs checks across borders. You’ll spot brand names like Plastomoll I, Polysorbate DAIB, and specialty catalog numbers when sourcing for industry or research. These names ease procurement by reducing confusion over grade or intended use, though end-users still rely on direct technical discussions to avoid mix-ups, especially in overlapping markets like plastics and cosmetics.
Safety & Operational Standards
A strong commitment to operational safety drives every stage of DIBA manufacture and handling. Chemical plants design dedicated transfer systems to prevent leaks. Long experience in facilities management teaches most safety lapses result from skipped steps or outdated gear. Workers suit up in protective gear, and storage takes place in cool, well-ventilated spaces, far from oxidizing materials. Safety drills focus on spill response and correct neutralization procedures, as high temperatures during production risk sparking fires. Industry-wide, suppliers post regular hazard updates, reflecting research on long-term low-level exposure and the need for regular air quality checks around process areas. Routine inspections remind us that what seems stable in the lab sometimes behaves differently under factory stress, especially at high production rates.
Application Area
You’ll see DIBA spread across industries—plastics, cosmetics, paints, adhesives. Its softening ability makes it a leading plasticizer in flexible PVC, which ends up in wire insulation, faux leather, and food packaging. Cosmetic chemists select DIBA for lotions, sunscreen, and shampoos because it offers a velvet feel without blocking skin’s natural breathing or causing slips on application. Printing inks, lacquers, and industrial lubricants lean on DIBA’s compatibility to maintain spreadability and prevent buildup on rollers or brushes. Over the years, I’ve watched producers chase ever-milder plasticizers to meet better skin tolerability and environmental standards, and DIBA often qualifies thanks to its low toxicity in real-world conditions, unlike phthalate esters that fell out of favor. Emerging industries like medical device manufacturing and specialty adhesives now see DIBA as an easy-to-manipulate starting point for technical blends that demand minimal migration.
Research & Development
DIBA receives ongoing R&D attention, mainly in search of greener synthesis and replacement for legacy phthalates. Scientists now explore bio-based routes, using renewable-feedstock alcohols or biogenic adipic acid to lower the product’s carbon footprint. Some studies track DIBA’s function as a carrier subject for actives in transdermal patches, looking for longer-lasting release with low irritation. Lab teams run repeated formulation trials to keep down volatility, aiming for formulations that last on the shelf even when shipment delays heat up warehouse docks. The regulatory climate asks for transparency, so a lot of collaborative industry-academia data collection goes into annual safety reviews and white papers for agencies. Taking part in this world, I’ve seen first-hand how minor process shifts—switching a catalyst, fine-tuning wash steps, or switching cleaning agents—bring down operational costs and environmental impact just as much as whole new reactor systems.
Toxicity Research
Over decades, public and private labs have run animal studies and cell-culture screens to track DIBA’s toxicity. Oral and dermal LD50 values both rank high, marking DIBA as much less acutely toxic than heavier phthalates. Skin patch tests run by cosmetic firms show low irritation in most human volunteers, though a few report transient redness, pushing brands to tighten on-label warnings. Chronic studies sift through effects on endocrine function and reproductive health, long a hot spot for plasticizers. From my point of view, regulators move slow only because new impurities or breakdown products occasionally slip through, forcing risk assessments to assume worst-case. DIBA hasn’t shown strong links to major health hazards at environmental or occupational exposure levels, though caution remains, especially in products with long-term skin contact or routes for accidental ingestion.
Future Prospects
Looking ahead, DIBA stands a good chance of growing into broader markets. Tech innovators seek out its use in nanomaterial dispersions and sustainable packaging films that demand both softness and clarity. With mounting restrictions on phthalates and mounting interest in renewable materials, DIBA faces real competition from both novel bio-plasticizers and traditional glycols. The regulatory push demands cleaner, transparent manufacturing, so supply chains may shift upstream, closer to major bio-feedstock producers. As digital tracking tools make every step traceable, buyers want assurance on source, purity, and toxicity, raising the bar on technical support and documentation. If ongoing research continues to prove DIBA’s safety and performance, and if companies press ahead with greener process routes, I expect DIBA to remain a steady favorite among flexible, low-hazard plasticizers and emollients for years to come.
The Role of Diisobutyl Adipate in Daily Products
Step into any drugstore and scan the shelves—from lotions to sunscreens and even some makeup products—and you'll probably run into diisobutyl adipate somewhere in the ingredient lists. This clear, oily liquid doesn’t get the attention that “natural” oils or vitamins attract, but it quietly does a lot of heavy lifting. In cosmetics and skincare, it works as an emollient and softener. This gives creams their smooth, spreadable feel and leaves skin feeling supple without a greasy aftertouch. Cosmetic chemists look for ingredients that help products feel pleasant—and diisobutyl adipate checks that box, improving both texture and comfort for the user.
Sun Protection Formulas and Why It Matters
Reliable sunscreen is a must. In those formulas, diisobutyl adipate helps dissolve UV filters and keeps everything from separating, making sure protection applies evenly. If you’ve spent years dealing with thick, sticky sunscreen, you start to notice which ones have a velvety finish and rub in easily. The ingredient lets sunscreen sit well on the skin, helping people actually want to use it every day. Good usability in sun products isn’t just a luxury—it helps regular sunscreen users avoid burns, sun damage, and skin cancer risk.
Going Beyond Skin Care—Household and Industrial Uses
Household cleaners, lubricants, and paint often contain plasticizers that keep formulas flexible without cracking or separating. Diisobutyl adipate helps do that job. In plastics and coatings, it makes finished products less brittle and more bendable. Window seals, automotive parts, and even flooring often include plasticizers like this one. Having grown up around older homes where cracking or shrinking flooring showed up in winter, I’ve come to respect materials that help things last longer and resist weather.
Safety and Environmental Perspective
Many parents want reassurance about the chemicals in products their families use. Diisobutyl adipate earns a pretty solid safety profile when compared to other plasticizers like phthalates, which have fallen out of public favor. Regulatory agencies in Europe and North America have reviewed its use and set restrictions where needed, but it doesn’t carry the same red flags around toxicity or hormone disruption. Still, the chemical industry—like any other—can’t ignore growing demands for greener options. Shoppers today expect more transparency and safer alternatives. Ongoing studies and best practices for disposal and manufacturing will answer those concerns over time.
Finding Smarter Solutions
As workplaces and homes look for planet-friendly choices, chemists keep working on alternatives that do what diisobutyl adipate does, only with lower environmental costs. Brands already test biodegradable or plant-based plasticizers. Supporting research into safer or renewable ingredients makes sense for both health and the planet, and manufacturers feel growing pressure to back up their claims. In the meantime, understanding what goes into the products we reach for every day—like diisobutyl adipate—helps everyone make smarter choices at the store and at home.
Everyday Products and Unfamiliar Ingredients
Diisobutyl adipate pops up in a surprising number of household and personal care items. If you’ve used sunscreen, makeup, or even some body lotions, you’ve probably smeared a bit of it on your skin without realizing it. It acts as an emollient, which helps skin smooth out and lets products spread better. Most folks don’t stop to search up every scientific term on the back of a bottle. Still, questions about safety come up over and over again, especially as people take a closer look at what’s inside the things they use every day.
What Science Says About Diisobutyl Adipate
Research has been thorough on this one. The Cosmetic Ingredient Review panel gave it a green light after looking at both animal and human studies. Their findings showed low risk of irritation or toxicity, even after repeated use. The U.S. Food and Drug Administration lists it as generally recognized as safe for use in food packaging, which speaks volumes since those standards tend to be strict. The European Chemicals Agency reaches a similar conclusion, so this isn’t just a North American thing.
I spent two decades working with dermatologists and formulating skincare products, so I’ve heard plenty of stories. Most people with average skin don’t react at all; redness or itching is rare and, when it comes up, usually ties back to another ingredient in the mix rather than diisobutyl adipate itself.
It Pays To Read Labels
Allergic reactions and sensitivities happen. No product suits everyone. Some people break out at the drop of a hat; others can use nearly anything without a hint of trouble. Patch testing a new product on a small area always seems wise, especially for someone with a known history of hypersensitivity. Looking for fragrance-free or hypoallergenic versions may also help those with delicate or eczema-prone skin.
Why Transparency Builds Trust
Concerns about chemicals grow bigger every year. Shoppers have a right to know what they’re using. Companies do a better job now of sharing ingredient origins and explaining why each one is used. Still, it feels better talking to a brand that offers clear answers and reliable science instead of empty promises. I’ve worked with labs that made every test result easy to find and even encouraged consumer questions, and I saw trust grow as a result.
Room for Improvement
Demand for alternatives with a fully plant-based pedigree keeps rising. Some companies already pivot to more natural emollients, driven by sustainability goals as much as safety. Few of these alternatives outperform diisobutyl adipate on all fronts, especially in sun protection formulas where performance means skin health. More research into bio-based replacements could give plenty of peace of mind long term.
Practical Steps for Everyday Skin Health
Protecting skin isn’t just about avoiding one ingredient. Applying sunscreen, steering clear of harsh detergents, and using gentle cleansers often matter more. Anyone unsure about a product—maybe the label raises questions or past reactions give pause—should reach out to a dermatologist for answers tailored to their own skin. That way, doubts can be put to rest without guesswork.
Science points out that diisobutyl adipate ranks as a safe ingredient for those without certain sensitivities. Personal habits, honest brands, and a little knowledge go a long way toward comfortable, healthy skin.
Flexible Plastics on Store Shelves
Spend any time in a supermarket and you’ll spot Diisobutyl Adipate at work, even if you’ve never heard the name before. It turns up in plastic wraps, flexible packaging, and squeeze bottles. This chemical helps turn otherwise stiff plastics into soft and pliable products that handle bending, squeezing, and folding. Without such plasticizers, we would deal with brittle materials that crack at a little pressure—think grocery bags that break after the third can of beans. In an age when convenience and food safety matter, soft plastics owe a lot to this ingredient.
Cosmetics: Smoother Lotions and Creams
Diisobutyl Adipate often slips into lotions, sunscreens, and makeup for reasons anyone who uses skincare can appreciate. Many moisturizing creams promise a non-greasy feel and a light touch. This is tricky, since mixing oils and creams typically brings that heavy or sticky feeling. Yet, this chemical adds that sought-after silkiness, helping products spread across the skin without turning oily. This role goes beyond just comfort—the lightweight texture encourages people to actually use sunscreen more often, which can fight premature aging and lower skin cancer risk. If you check the ingredient list on newer broad-spectrum SPF products, you’ll likely spot it, because it works with both the active sunscreen ingredients and skin.
Industrial Uses: Lubricants and Processing Aids
Factories and workshops often rely on lubricants to keep moving parts safe from wear and sticking. Diisobutyl Adipate shows up in a number of industrial oils, supporting smooth machine performance. It blends well with base oils while reducing friction and keeping equipment running day after day. This extends the working life of machines—no small feat where repairs can disrupt business or production for hours. Equipment downtime hits output and profits, so an effective lubricant can pay for itself many times over. Engineers at companies that mold, extrude, or coat plastics appreciate the help this material offers, especially when shifting product lines or working with recycled materials.
Inks and Coatings: Consistent Printing and Clean Results
The ink that fills up pens and printers takes more than color to flow properly. Diisobutyl Adipate tailors the consistency of inks for markers, offset presses, and packaging. It makes inks smoother, reduces unexpected smearing, and keeps pigment from clumping. Signage, labels, and commercial packaging benefit from the stability this brings, which helps logos and instructions stay clear through handling and shipping. Art supply brands use it in their professional lines when reliable results matter most. In coatings, it enables glossy surfaces without excessive stickiness or odor, offering a better user experience and longevity for painted or coated items.
Environmental and Safety Considerations
Chemicals in consumer goods have come under increasing scrutiny. Diisobutyl Adipate currently holds a solid safety profile among regulators, but industry trends push for further transparency and cleaner alternatives when possible. Many cosmetics firms have published assessments confirming its low irritation risk and lack of major toxicity. Still, demand for biodegradable and renewable-based plasticizers is rising. Researchers and manufacturers look for ways to offer the same softening effects and application benefits while shrinking the chemical footprint.
Looking Ahead: More Than a Supporting Role
If you've pulled cling film over leftovers, smeared sunblock before an afternoon run, or cracked open a highlighter, you've benefited from the flexible work of Diisobutyl Adipate. Many industries depend on its versatility, but as safety and sustainability grow in importance, this ingredient sits at a crossroads. Some manufacturers are experimenting with biobased alternatives, green chemistry routes, and more efficient production to balance practicality with environmental responsibility. Even though it often goes unnoticed, the science behind everyday convenience continues to evolve, often in response to what consumers and regulators demand.
Getting Familiar with Diisobutyl Adipate
There’s a quiet importance to knowing what’s in the stuff we use every day. Diisobutyl adipate turns up in places folks rarely notice—like some cosmetics and stretches of plastic wrap on your lunch. Its chemical formula is C16H30O4. This isn't some obscure fact; it tells a story about the compound’s makeup and behavior.
The Reason Formula Matters
In practice, understanding this formula means understanding what the compound brings to a table, beyond just being an ingredient listed in small print. With sixteen carbons, thirty hydrogens, and four oxygens, this molecule offers real flexibility—the kind manufacturers crave when designing materials that bend or flow without snapping or cracking. It’s got two isobutyl groups attached to adipic acid, shaping the way it interacts with plastics, lotions, and more.
Having worked in fields that cross both product design and chemical safety, I’ve come across this compound most when trying to dial in the right blend for something like a vinyl glove or sunscreen. Too rigid and the material falls apart; too greasy and nobody wants to touch it. Diisobutyl adipate delivers a sweet spot, thanks to the structure its formula reveals.
Concern for Safety and Health
Any time chemicals seep into consumer goods, people start to ask: Is it safe? That’s not just a paranoid impulse; we’ve lived through scares with plasticizers and additives before. Luckily, what current studies say about diisobutyl adipate is reassuring. Regulators in the US and Europe looked hard at it. Used at allowed levels, no major health hazards turn up—though researchers keep tabs for long-term shifts, especially as we learn more about how small exposures can add up.
In real life, workers mixing large vats of this stuff still need gloves, goggles, and good ventilation. It's not about fearmongering; it's about practical respect, both for the science and the people on the factory floors.
Where Waste Goes
Anyone who cares about what goes down the drain wonders what happens next. Diisobutyl adipate breaks down pretty well in the environment, which is less true for some of its chemical cousins. Still, disposal takes thought. Factories can’t just dump leftovers. Treatment systems catch most of it before it reaches rivers or landfills—and responsible companies test for traces to stay ahead of pollution problems.
Waste doesn't just disappear. My experience working with environmental engineers taught me that small oversights can balloon into bigger headaches, both for companies and nearby communities. Testing, updating disposal routines, and listening to environmental scientists help avoid mistakes that cost more in the long run.
Better Choices Ahead
The push toward greener, safer chemicals isn’t slowing down. Plant-based alternatives may someday replace compounds like diisobutyl adipate, making future plastics and cosmetics gentler on both people and the planet. Until then, knowing that formula, C16H30O4, isn’t just classroom trivia. It’s a key to understanding how decisions in a lab or factory echo into products and places we see every day.
Straightforward Storage Strategies
Anyone who works with chemicals knows it’s tempting to treat some materials like background noise—especially common plasticizers like Diisobutyl Adipate. Yet ignoring the details around storage can cause headaches and unnecessary safety issues down the road. Diisobutyl Adipate flows as a clear liquid, doesn’t give off much of a smell, and hasn’t made headlines for dramatic accidents. That’s no excuse to relax standards or stack drums in any old shed.
Heat and light tend to degrade many plasticizers, including this one. Storing the liquid in tightly sealed containers, away from sunlight and out of the reach of heat sources, helps preserve its integrity and reduces the risk of material breakdown. Steel drums with secure gaskets keep the product clean and prevent air or moisture from getting inside. Anyone who’s ever opened a compromised container after a hot summer knows how unpleasant it is to throw out ruined material and spend hours cleaning a sticky mess.
A controlled temperature, ideally below 30°C, prevents Diisobutyl Adipate from thinning out and leaking or from interacting with other products nearby. A spill near oxidizing chemicals could trigger heat or fumes—not catastrophic, but enough to shut down operations while everyone puts on their PPE and cleans up.
Daily Handling in the Real World
Getting complacent with chemical transfers has never ended well for anyone. When filling smaller containers or reloading pumps, careful handling matters. Gloves, splash goggles, and protective clothing aren’t overkill—they’re just common sense. The substance won’t burn skin on contact, but it can irritate, and folks get careless and rub their eyes or eat lunch right after a spill. Strong ventilation helps with any vapors, especially in small rooms or near open drums. No one wants to head home with a headache they can’t explain.
Teams should practice good housekeeping habits, cleaning up any droplets or residue right away. Slick floors lead to broken bones and worse. Any rags or materials used in the cleanup process should go straight to dedicated chemical waste bins—not into general trash—since slow leaks lead to surprises long after everyone clocked out for the day. Keeping an up-to-date Safety Data Sheet (SDS) on hand provides crucial information if something goes wrong. The details on fire risk, first aid, and emergency contacts aren’t just there to check a regulatory box; they save time in real emergencies.
Supporting Workers and the Environment
Organizations owe it to their teams and the local community to minimize risk at every turn. Routine training on correct storage and careful transfer pays off. Near-misses deserve as much attention as real accidents—a quick toolbox talk about a near spill often stirs more awareness than a formal training video. Asking folks on the floor for feedback on what’s working and what isn’t can catch problems before they reach management.
Many industries face local fire code or environmental regulations on solvents and plasticizers. Exceeding the minimum keeps everyone safe and shows real respect for the materials and the people handling them. Spills stick around in the soil, and no business needs an EPA fine or a knock on the door from environmental inspectors. Double-checking the secondary containment systems every quarter—rather than waiting for a real leak—can save thousands in clean-up costs and plenty of stress.
Responsibility for safe handling starts with personal accountability. Everyone benefits from an environment where open discussions about chemical safety happen every week, not just after something goes wrong.
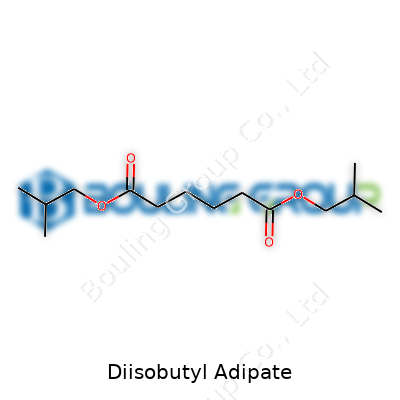
| Names | |
| Preferred IUPAC name | bis(2-methylpropyl) hexanedioate |
| Other names |
Adipic acid diisobutyl ester
Diisobutyl hexanedioate DIBA Hexanedioic acid diisobutyl ester Adipic acid, bis(2-methylpropyl) ester |
| Pronunciation | /daɪˌaɪsəˈbjuːtɪl ˈædɪpeɪt/ |
| Identifiers | |
| CAS Number | 141-04-8 |
| Beilstein Reference | 1209430 |
| ChEBI | CHEBI:88993 |
| ChEMBL | CHEMBL3634073 |
| ChemSpider | 57014 |
| DrugBank | DB16647 |
| ECHA InfoCard | ECHA InfoCard: 100.009.041 |
| EC Number | 203-090-1 |
| Gmelin Reference | 60761 |
| KEGG | C19699 |
| MeSH | D02.241.081.220.110.700 |
| PubChem CID | 31258 |
| RTECS number | AF9650000 |
| UNII | P0A3332C8G |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | DTXSID9040957 |
| Properties | |
| Chemical formula | C14H26O4 |
| Molar mass | 286.42 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.947 g/cm3 |
| Solubility in water | Insoluble |
| log P | 3.61 |
| Vapor pressure | 0.01 mmHg (25 °C) |
| Acidity (pKa) | 10.33 |
| Basicity (pKb) | Product has no basicity (pKb) as it is an ester and does not act as a base. |
| Magnetic susceptibility (χ) | -7.71×10⁻⁶ |
| Refractive index (nD) | 1.436 |
| Viscosity | 15.1 mPa·s (25°C) |
| Dipole moment | 2.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 687.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -902.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3896.7 kJ/mol |
| Pharmacology | |
| ATC code | D11AX22 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H317: May cause an allergic skin reaction. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. IF ON SKIN: Wash with plenty of water. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 145°C |
| Autoignition temperature | 355 °C |
| Lethal dose or concentration | LD50 oral rat > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Diisobutyl Adipate: "greater than 64 g/kg (oral, rat) |
| NIOSH | WA7870000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Diisobutyl Adipate: Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Dibutyl Adipate
Diethyl Adipate Dimethyl Adipate Diisobutyl Sebacate Diisobutyl Phthalate |

