Dimethyl Carbonate: A Comprehensive Commentary
Historical Development
Long before sustainability buzz filled chemistry labs and boardrooms, researchers eyed Dimethyl Carbonate as an answer to cleaner manufacturing challenges. Years ago, processes like phosgenation dominated the scene—risky, toxic, almost unavoidable. Inventors in the late 20th century crafted alternative approaches, leading to the advent of non-phosgene methods using carbon dioxide and methanol. This marked a significant shift, not only in laboratory innovation but also in the broader landscape of chemical industry safety and environmental responsibility. Companies in Japan and Europe emerged as leaders in scalable production, driven by mounting regulatory pressure and consumer demand for safer, green chemicals.
Product Overview
Dimethyl Carbonate serves as more than just a chemical intermediate. In my years consulting for specialty solvent manufacturers, I noticed its growing popularity where versatility and lower toxicity matter. The molecule, known in the trade as DMC, bridges needs across electronics, paints, adhesives, and even pharmaceuticals. From fuel additives to battery electrolytes, it steps in where petrochemical-based options stumble, offering a nearly odorless, biodegradable solvent that holds potential to limit workplace exposure risks.
Physical and Chemical Properties
DMC appears as a clear, colorless liquid, typically stored in stainless steel drums or bulk ISO tanks. Its molecular formula, C3H6O3, features two methyl groups and a carbonate functional group, giving it a distinct chemical profile. With a boiling point around 90°C, moderate vapor pressure, and a specific gravity close to 1.07, the handling feels familiar to those who have worked with esters. What always strikes me is its high solvency power for polar and non-polar substances—a rare advantage, especially in coatings labs where tailorable solvent blends are required.
Technical Specifications and Labeling
On specification sheets, one typically finds purity levels above 99.5%, residual water kept below 0.05%, and acid numbers tightly controlled. Producers place strong emphasis on compliant labeling, noting hazard statements such as ‘flammable liquid’ and ‘causes serious eye irritation’. Shipping requires adherence to UN1161 regulations under the IMDG and DOT codes. From years in regulatory compliance, I can say that proper labeling—detailing batch numbers, manufacturing dates, and disposal guidelines—prevents confusion during warehousing and handling.
Preparation Method
Industrial preparation largely relies on catalytic oxidative carbonylation of methanol using CO and oxygen, catalyzed by copper or noble metal complexes. Formerly, phosgene-based methods posed ethereal risks—but their phase-out opened room for safer, greener synthesis. Chemical engineers often tweak operating pressures and temperatures—usually 90°C to 120°C under mild pressure—to maximize yields and curtail side products like dimethoxy methane. In pilot plants, recapturing unreacted gases and purifying the final product with fractional distillation helps maintain cost-effectiveness and consistency.
Chemical Reactions and Modifications
DMC’s reactivity stands out in transesterification processes. In my lab, we preferred it over methyl chloride as a methylating agent due to safer handling and less toxic byproducts. It reacts readily with phenols, amines, and alcohols, generating valuable methyl ethers and carbamates—key ingredients for polycarbonate resins, pesticides, and pharmaceuticals. Its ability to replace phosgene in polycarbonate synthesis translates to enormous gains in plant safety and waste reduction, which always resonated with process engineers focused on cleaner chemistry.
Synonyms and Product Names
Most chemical catalogs list DMC under names like ‘carbonic acid dimethyl ester’, ‘methyl carbonate’, or simply ‘methyl carbonate ester’. In various jurisdictions, registration numbers like EC 210-478-4 or CAS 616-38-6 appear on certificates of analysis. Commercial brands marketed as ‘Ecoline DMC’ or ‘GreenSolv Methyl Carbonate’ often denote high-purity or eco-friendly product variants—something clients in paints and electronics find attractive when marketing to eco-conscious buyers.
Safety and Operational Standards
Handling calls for standard flammable liquid precautions—spark-proof tools, grounded drum pumps, and proper ventilation in filling rooms. I’ve seen too many operators underestimate its potential as both an irritant and a fire hazard. Chemical splash goggles and nitrile gloves are part of the minimum kit. Storage away from strong oxidizers or acids reduces risks of unwanted polymerization or hazardous releases. Spills call for absorbent sand and prompt containment, as with similar solvents.
Application Area
Dimethyl Carbonate features prominently in batteries as an electrolyte solvent. My experience with electronics manufacturers revealed its quick adoption in lithium-ion and lithium-polymer battery formulations, due to low viscosity and electrochemical stability. Coatings engineers use it to formulate low-VOC paints. Fuel technologists blend it with gasoline to raise oxygen content and reduce emissions. Pharmaceutical labs favor it for methylation steps, reducing reliance on more dangerous reagents. Emerging applications always stem from greener product lines—bio-based plastics and performance solvents in eco-friendly adhesives.
Research and Development
Academic journals regularly publish work on catalyst development, carbon capture integration, and route optimization. At a recent conference, I watched groups present microreactor technologies and continuous flow systems to cut waste and save energy. Collaboration between industry and academia focuses on lifecycle analysis, reusability of reaction byproducts, and integration with bio-feedstocks. Such research gains traction, especially as government grants increasingly favor sustainable chemistry initiatives.
Toxicity Research
Toxicological studies rate DMC as low hazard compared to traditional alkylating agents. Acute exposure mainly causes eye or skin irritation; severe cases result from large vapor concentrations in unventilated spaces. Animal studies reveal no mutagenic or carcinogenic effects at typical workplace exposure levels. Regulatory reviews by agencies like ECHA and US EPA reflect this, classifying it as low risk for chronic health concerns when handled properly. In my tours of manufacturing sites, well-managed exposure controls helped prevent incidents—showing the importance of good industrial hygiene.
Future Prospects
The trajectory looks bright as electric vehicles and green innovation drive demand for better battery electrolytes and clean fuel additives. DMC’s role as a sustainable alternative keeps growing as companies work to decarbonize supply chains. Policy levers, such as stricter VOC limits and phosgene phase-outs, support wider adoption in paints, coatings, adhesives, and plastics. Researchers aim for more efficient synthesis pathways using renewable CO2, which could cut costs and environmental footprint at the same time. As society shifts toward a circular economy, materials like Dimethyl Carbonate step into the spotlight—and the need for robust research, responsible manufacturing, and sound safety practices becomes even clearer.
Chemical Production: A Cleaner Approach
Dimethyl carbonate carries a reputation as a safer alternative in the chemical world. For years, manufacturers saw the dangers posed by toxic chemicals like phosgene. Many have switched to dimethyl carbonate for making polycarbonate plastics. These plastics end up in everything from car headlights to reusable water bottles. Using this compound means workers and communities face fewer risks from chemical leaks and environmental spills. I remember meeting a plant operator who explained how switching to dimethyl carbonate transformed the safety culture in their small-town factory.
Solvent in Industry Labs
Walk into a paint lab or an adhesive production line, and chances are you’ll find bottles of dimethyl carbonate. People look to it as a green solvent. Painters, engineers, and chemists use it to dissolve materials without the headaches associated with more dangerous compounds. For instance, methyl ethyl ketone often sits in the news as a harmful pollutant. By swapping in dimethyl carbonate, workplaces cut down on fumes—and staff stress less about exposure. In my own time helping a friend fix up old furniture, paints thinned with this compound made the job much less hazardous and a lot more pleasant.
Batteries and Fuel Additives
Anyone who’s cracked open a modern phone or electric car knows batteries play a huge role in daily life. Dimethyl carbonate also finds a home here. Battery makers blend it into electrolytes for lithium-ion cells. Without the right solvent, these batteries lose performance and lifespan. I’ve listened to engineers explain how the right chemical mix keeps batteries reliable in harsh winters and blazing summers. This compound also fits into the push for cleaner fuels, serving as an oxygenate in gasoline. Adding it improves combustion and cuts down harmful exhaust. Neighbors living near busy streets have noticed cleaner air after regulators pushed for these blends in city gasoline.
Pharmaceuticals: A Role in Medicine
Dimethyl carbonate quietly helps produce many prescription drugs. Its combination of low toxicity and reactivity lets scientists assemble complex molecules without dangerous leftovers. At the small-town pharmacy where I worked my first summer job, staff often chatted about how raw materials reach their shelves. Knowing that drug makers now turn to dimethyl carbonate gives confidence that prescriptions are safer from contamination risks.
Environmental Benefits and Solutions
Switching to dimethyl carbonate means more than boosting production or cutting costs. It represents part of the environmental cleanup people have been demanding. The EPA points to this compound as a preferred alternative, thanks to its low toxicity and biodegradable nature. Still, wider adoption doesn’t solve everything. Communities need clear data about any leaks or long-term health effects. Strong workplace training, regular environmental checks, and investment in greener disposal methods can help build trust. Leaders in industry should partner with public health experts and local governments to track air and water quality as use of dimethyl carbonate grows.
Responsible Growth Matters
People want goods that don’t jeopardize their safety or the world around them. Dimethyl carbonate shows how smart choices in chemistry support both innovation and responsibility. By focusing on cleaner alternatives and ongoing oversight, communities benefit now and set up a healthier future.
Finding Better Chemicals for the Planet
Green chemistry means using chemicals that cause less harm to people, animals, and the environment. Companies search for safer alternatives because the old chemicals they relied on often pollute air, water, and soil. Dimethyl carbonate (DMC) is often pointed out by the chemical industry as a breakthrough. Some call it “a green chemical” because its production skips chlorine, and its byproducts are less toxic compared to old solvents. Many tout its lower toxicity and easier handling. But does DMC deserve its eco-friendly reputation?
What Dimethyl Carbonate Replaces
Solvents like phosgene and methyl chloroform have made headlines for the mess they leave behind. Phosgene, in particular, caused disasters. DMC doesn’t release the same level of toxic emissions, and it won’t generate hydrochloric acid as a byproduct. Think about factories that produced things like polycarbonates or fuel additives; by switching to DMC they can lower risks for workers and reduce air pollution, both real improvements. People living near these places might breathe easier—literally.
How DMC Gets Made
Most producers create dimethyl carbonate from methanol and carbon monoxide, using catalysts that don’t rely on dangerous metals. The process uses carbon monoxide or sometimes captures carbon dioxide—gases everyone wants to keep out of the air. Catching carbon dioxide to make something useful is a good idea and could offer a way to reduce greenhouse gas buildup, at least on paper.
Yet, factories need large volumes of methanol, usually made from natural gas. Turning natural gas into methanol burns a lot of fossil fuels. If that process doesn’t move to renewable sources, calling DMC green feels incomplete. DMC made from biomass or using carbon dioxide pulled from the air would improve its environmental score, but that’s still a small fraction of production.
Working with DMC in Real Life
I’ve seen labs where workers treat DMC with respect, but not the same fear as older solvents. Its vapors can irritate eyes and lungs, but spills pose fewer dangers. Disposal doesn’t create legacy toxins the way chlorinated solvents do.
On the flip side, if care slips during production or transport, DMC can still add to local pollution. The chemical breaks down into methanol and carbon dioxide—methanol by itself poses a real hazard to aquatic life.
So, Is DMC Environmentally Friendly?
People want an easy answer: yes or no. The truth runs in shades of gray. With responsible sourcing and controlled use, DMC may help industries cut down risks for workers and communities. Its production can be greener if it leans into cleaner sources of methanol. For users needing to replace dangerous solvents, DMC does bring safety benefits and less environmental baggage, but energy and resource use still matter.
If policymakers and companies committed to renewable feedstocks, DMC could be a cleaner bridge from old pollution-heavy chemistry to something that sits lighter on the planet. Innovation from startups and pressure from buyers can nudge the chemical industry in a better direction.
Room for Improvement
Better sourcing stands out as a fix with the most impact. Tech advances in carbon capture or methanol from captured carbon could push DMC over the edge, making it truly green. As with so many “green” options, details about how and where it’s made shape the story.
Dimethyl Carbonate: A Closer Look at the Chemistry
Dimethyl carbonate shows up in a lot of places—from safer solvents to green fuel additives. On paper, scientists and researchers know it as C3H6O3. The name spells out its basics: two methyl groups (the “dimethyl” part) hooked onto a carbonate core. The shape might not grab your attention at first, but behind that simple formula sits a structure that matters a whole lot to both environmentalists and engineers.
Its molecular structure has a central carbonate group (CO3) where the carbon atom bonds to three oxygen atoms. Instead of what you'd find in regular carbonates, here two of those oxygens are attached to methyl groups (–CH3). Lining out the formula, the molecule looks like this: CH3OCOOCH3. Some draw it to show a straight line—methyl, then an oxygen, next to a central carbonyl, then another oxygen, and another methyl at the end. You end up with two short “arms” stretching out from the carbonate center, both capped with that familiar methyl group.
Why Structure Matters: More Than Just Chemistry
You might ask, what difference does this exact molecular layout make in real life? Turns out, a lot. The two methyl ends give it an edge over plain carbonates, both when it comes to how easily it evaporates and how it mixes with other chemicals. This opens the door for it to replace more toxic substances like phosgene or methyl chloroformate. For decades, industries have leaned on those nastier versions because they work. Dimethyl carbonate lets manufacturers keep efficiency high while cutting back on hazardous byproducts.
I've seen chemical plants adopt safer solvent options not just to please regulators, but to seriously cut down industrial accidents. Anyone who’s ever watched a heavy-duty sealant or paint thinner eat through a pair of gloves knows the importance of safer alternatives. Dimethyl carbonate’s relatively mild toxicity profile comes from its structure—the way the carbonate center links up to those methyls means it acts as a great solvent, yet doesn’t linger in the environment or the body.
Supporting Sustainability in Industrial Chemistry
The push for sustainable chemistry has never been about switching one molecule for another on paper. It’s about making processes safer for both workers and the world outside the factory fence. With dimethyl carbonate, the structure allows it to serve as a raw ingredient (a “green” reagent) for polycarbonates and pharmaceuticals. This means fewer harsh chemicals needed on site and a smoother transition to closed-loop, less-polluting manufacturing.
Academic studies have repeatedly shown dimethyl carbonate decomposes into carbon dioxide and methanol under normal conditions. Its low toxicity shows up not only in experiments but in workplace stats—fewer health incidents, fewer task interruptions. That’s a clear win where safety is concerned.
Turning Knowledge Into Practice
Changing out old industrial habits is never a plug-and-play operation. I’ve seen facilities do small-scale pilot runs with dimethyl carbonate before rolling it out plant-wide, just to make sure everything fits. Those first-hand experiences tell us that understanding the chemistry—right down to the formula and atomic connections—is the key to getting results that stick. The more decision-makers grasp how the formula enables more responsible handling and cleaner output, the more likely they’ll keep this molecule in their lineup for years to come.
The Real Risks People Miss
Few folks outside the lab crowd know much about dimethyl carbonate. It’s a chemical with all kinds of uses—paint thinner, cleaner, and a key piece in making polycarbonate plastics. Even so, some workers see clear liquid in a bottle and get lax. That gets people hurt. To avoid trouble, a good grip on regular safety steps makes all the difference.
Air You Actually Want to Breathe
Breathing in fumes is no joke, even if the stuff doesn’t smell all that strong. Eye irritation, dizziness, and a wicked headache sneak up fast. My own early gig involved bottling chemicals, and I learned the hard way that fans and open windows don’t move enough air out. Real ventilation—fume hood, dedicated extraction, fresh air swaps—stops that sick feeling before it starts. Folks who work with it every day need to respect what regular ventilation does. It’s not just a box on a checklist; it’s the difference between a productive shift and a trip to the infirmary.
Gloves Aren’t Optional
Skin absorbs dimethyl carbonate more than people think. Gloves should be ready before the cap even comes off. Skip the thin stuff—tear-resistant nitrile or butyl works best. I’ve seen coworkers wipe spills with a bare hand or, worse, a thin rag. The chemical dries the skin, brings on rashes, and, over time, does more if you keep letting it slide. Long sleeves, tight cuffs, and a good habit of double-checking gear matter. Goggles belong in the same bin—nothing’s more memorable than the day your vision goes blurry from a careless splash.
Storage Habits Separate Newbies from Pros
Leaving dimethyl carbonate out on a shelf or near heat sources courts disaster. It evaporates, builds pressure in closed bottles, and leaks if a seal loosens up. A steel drum bulging at the seams taught me the value of storing containers upright, in cool, dry rooms with lots of airflow. Labels wear out, and the sharpie fades, but nobody forgets the smell of something leaking where it shouldn’t be. Lock up containers tight, keep flammable stuff away, and check for rust before every shift.
Quick Response to Spills
A chemical spill doesn’t wait for a supervisor. Every worker needs to know their spill kit and how to use it. Granular absorbent, neutralizer, and thick gloves belong next to work benches. Once, an elbow bump sent a beaker flying, and the next five minutes made all the difference—spill contained, nobody injured, operation kept on track. Cleanup routines beat panic, and a well-stocked kit costs a lot less than missed work and angry neighbors.
No Shame in Speaking Up
In my early days, voicing concern about leaks or improper gear earned side-eye from old-timers, but people notice who goes home safe and who doesn't. Safety meetings and quick refreshers before new projects help keep everyone honest. Simple conversations go a long way. If you see someone skipping steps, a quick reminder might keep them safe next time.
Everything comes down to habits. Want to handle dimethyl carbonate without hospital visits or ruined gear? Stick to ventilation, cover up, store it smart, and don't cut corners during cleanup. These rules root themselves in experience—ignore them, and the chemical will remind you to respect it.
Understanding Dimethyl Carbonate in the Real World
Dimethyl carbonate might sound complicated, but it shows up in a lot more products and places than most people notice. It finds its way into solvents, fuel additives, and even pharmaceutical production. What catches my eye as someone who’s handled chemicals is not just how it’s used, but how we deal with it behind the scenes: storage and transportation are the backbone of safety with this compound.
Conditions Matter: Why Simple Methods Fall Short
Experience around warehouses and shipping docks teaches one thing fast—the little details around chemical storage can make or break safety. Dimethyl carbonate catches fire more easily than water boils. Even a tiny leak near an ignition source can lead to disaster. So, storage calls for serious attention. Drums and containers need sealing—good, thick steel, no rust, nothing to corrode. Rooms or tanks should sit away from sunlight, with cool air and robust ventilation. It's not overkill; it's practical, and it keeps people alive.
Official data backs this up. The material’s flash point sits low, around 17°C (just over room temperature for many places), according to NIOSH and SDS guidelines. Factories and warehouses keep dimethyl carbonate below this threshold—some add alarms or sensors that catch dangerous vapors. It’s not just for large plants, either. Even small labs and companies handling less than a barrel need the same care because one accident can set off a chain reaction.
Label Everything and Train Everyone
It sounds basic, but every container needs a clear, correct label. No one wins with guesswork, especially with flammable stocks. Every employee stepping onto the property has to know what’s in each barrel. I’ve watched too many incidents come down to “I thought it was only water.” Sharing the right training makes sure people can spot trouble before it starts—big warning signs, real-world walk-throughs, and honest talks, not just paperwork.
Transport Has No Room for Assumptions
Getting dimethyl carbonate from one place to another takes more than hiring a truck and locking the doors. Department of Transportation regulations flag it as hazardous: only certain vehicles, with sealed compartments and no spark hazards, make the grade. Shipping manifests double-check what’s inside each container. It’s here that experience counts—drivers and handlers who understand chemical cargo treat the load with the gravity it deserves. On the rails, rules are even more strict: the tank cars sport special linings and pressure controls, with staff trained for leaks or emergencies.
One story sticks in my mind: years ago, a freight driver caught a whiff of something sweet—the telltale smell of dimethyl carbonate. He pulled over, checked the seals, and found a faulty drum before things got worse. Quick action kept the road safe, the cleanup quick, and the supply chain on track. This kind of vigilance needs reinforcing every single day.
Looking Ahead: Better Solutions
Stronger containers and better tracking technology offer a step forward. RFID tags track lot numbers and spot leaks or temperature swings before a person even enters the storage area. Remote monitoring can mean fewer surprises, and paired with consistent on-the-ground training, these shifts go a long way toward preventing incidents. But no high-tech fix works without basics—cool temperatures, dry conditions, proper labeling, and staff who know their stuff and stay alert.
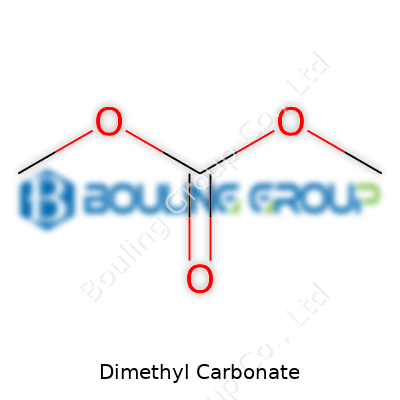
| Names | |
| Preferred IUPAC name | Methyl methyl carbonate |
| Other names |
DMC
Carbonic acid dimethyl ester Methyl carbonate Methoxycarbonyl methan Dimethyl ester of carbonic acid |
| Pronunciation | /daɪˈmiːθəl ˈkɑːbəneɪt/ |
| Identifiers | |
| CAS Number | 616-38-6 |
| Beilstein Reference | 2039152 |
| ChEBI | CHEBI:34797 |
| ChEMBL | CHEMBL50258 |
| ChemSpider | 5957 |
| DrugBank | DB11575 |
| ECHA InfoCard | 03aaf8c6-9a8a-484b-bc96-00b6be6c1536 |
| EC Number | 203-489-0 |
| Gmelin Reference | Gmelin Reference: 8716 |
| KEGG | C18673 |
| MeSH | D002576 |
| PubChem CID | 11715 |
| RTECS number | FG0350000 |
| UNII | E8VBO1805S |
| UN number | UN1161 |
| CompTox Dashboard (EPA) | DTXSID6020222 |
| Properties | |
| Chemical formula | C3H6O3 |
| Molar mass | 90.08 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 1.069 g/cm³ |
| Solubility in water | soluble |
| log P | -0.27 |
| Vapor pressure | 18.7 mmHg (20 °C) |
| Acidity (pKa) | 15.77 |
| Basicity (pKb) | Base: 3.65 |
| Magnetic susceptibility (χ) | -49.5e-6 cm³/mol |
| Refractive index (nD) | 1.369 |
| Viscosity | 0.585 mPa·s (at 25 °C) |
| Dipole moment | 4.55 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 178.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -604.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1785 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H319 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 17 °C |
| Autoignition temperature | 400 °C (752 °F; 673 K) |
| Explosive limits | 4.22% - 12.87% |
| Lethal dose or concentration | LD50 Oral Rat: 12,900 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Dimethyl Carbonate: Oral, rat: 13,000 mg/kg |
| NIOSH | FH9625000 |
| PEL (Permissible) | 100 ppm |
| REL (Recommended) | 0.5 ppm |
| IDLH (Immediate danger) | IDLH: 1,500 ppm |
| Related compounds | |
| Related compounds |
Methyl chloroformate
Dimethyl oxalate Diethyl carbonate Propylene carbonate |

