Formic Acid: A Deep Dive from Origins to Future Prospects
Historical Development
People started paying attention to formic acid after early scientists noticed its unique smell and sting in red ants. Around the seventeenth century, European chemists isolated this substance by distilling crushed ants, capturing a clear, sharp-smelling liquid. This odd approach didn’t last, as the industrial world kept looking for ways to make more of it, faster. By the late nineteenth century, laboratories swapped ants for chemical methods, using carbon monoxide and water at high pressures to deliver steady production. This marked a turning point: chemical industries could finally keep up with rising agricultural and textile demands. Over the years, process tweaks and better catalysts brought formic acid from a scientific curiosity into farms, factories, and preservation tanks, changing agriculture and chemical processing for good.
Product Overview
Some know formic acid best for its punchy odor and biting taste, but to those who use it in their daily work, it’s a basic necessity. Commercial formic acid usually comes as a clear, colorless, corrosive liquid. It finds its way into livestock feed, rubber manufacturing, leather tanning, and even beekeeping. Its role may sound scattered, but at its core, formic acid breaks down unwanted bacteria and helps preserve food and silage. Whether in 85% technical grade or as a diluted solution, it combines aggressive cleaning with practical disinfection, blending old world need for preservation with new world industrial processing.
Physical & Chemical Properties
Pure formic acid boils near 100°C and freezes at just under 9°C, which means it stays liquid across most farmyards and factory floors. Its molecular formula, HCOOH, packs a lot of punch in a small package. Unlike many acids, formic acid mixes pretty well with water, ethanol, and other solvents. This ability to slide into different mixtures lets industries swap out harsher chemicals for a more manageable option. Still, the substance eats at metals and softens plastics, so every working area needs proper containers and lines. Its vapor hugs the ground, pushing safety needs higher, especially in poorly ventilated rooms.
Technical Specifications & Labeling
Industry labels on formic acid bottles spell out danger and utility in bold letters. Key numbers on a label might read: minimum assay of 85%, water content below 15%, and iron content far below one hundred parts per million. Purity doesn’t just dictate where you use it—it can mean life or death in a confined workspace. Labels must list lot numbers, manufacturer names, and hazard symbols that speak plain truth about burns and corrosion risk. Compliance with international standards like REACH, OSHA, and the Globally Harmonized System keeps handlers aware of what’s inside each drum or tank, cutting down on accidents and waste in rough industrial settings.
Preparation Method
These days, factories turn out formic acid through hydrolysis of methyl formate. Chemists start by reacting methanol and carbon monoxide over a catalyst, usually sodium methoxide, to make methyl formate. Then, steam cracks methyl formate, releasing formic acid and methanol for recycling. Some older facilities still rely on caustic soda and chloroform in the “chloral” route, but this creates toxic byproducts and costs more on waste management. Modern methods slash raw material needs and pollution, meeting tough environmental quotas for Europe, North America, and East Asia.
Chemical Reactions & Modifications
Formic acid doesn’t sit idle in storage. Chemists count on it to reduce gold and silver salts, run esterification with alcohols, and tweak pH in dyeing and leather tanning. In the hands of a skilled operator, its high reactivity brings out new properties in synthetic fibers and pharmaceutical compounds. Reacting formic acid with sulfuric acid strips away water, leaving carbon monoxide behind. Under heat, it breaks down to hydrogen gas and carbon dioxide, fueling small-scale laboratory reactions. Few chemicals keep as many doors open, but few require as much respect at every handling point as formic acid.
Synonyms & Product Names
In catalogues and shipping manifests, formic acid might sneak by as “methanoic acid,” “hydrogencarboxylic acid,” or “E236” in food settings. Local traders and international suppliers sometimes call it “aminic acid.” In chemical supply houses, different brand names might suggest specialty grades—ultra-pure for labs, stabilized solutions for animal feed, technical grade for process industries. Users must dig through these product names to find the right purity, because cut corners can lead to halted production or sick animals when incorrectly sourced.
Safety & Operational Standards
Working with formic acid calls for more than gloves and goggles. Splash-proof aprons and tight-fitting respiratory masks keep workers safe from chemical burns and lung damage. Proper ventilation, leak detectors, and strict protocols cut down on exposure, especially during tank filling or waste transfer. Transport standards set limits on container types, stacking heights, and road temperatures. Comprehensive first aid training across the staff ensures quick, steady responses to even small spills or burns—it’s not just good management, but a necessity for keeping insurance costs down and worker confidence high.
Application Area
Farms use formic acid to fight bacteria in silage, extend shelf life, and trim down on antibiotics in livestock feed. Tanneries reach for it to pickle and preserve animal hides before processing them into soft leather goods. Textile factories rely on its acidic bite to balance dyes and set color on natural or synthetic fibers. Beekeepers use it in vapor or pad form to target mite infestations, supporting hive health. The rubber industry adds it during latex coagulation. Pharmaceutical labs push its limits in drug synthesis and sterilization, while food technologists remember its E236 number on preservative labels—always under strict dose controls.
Research & Development
Teams in universities and industrial labs keep stretching what formic acid can do, focusing on greener production, catalyst recycling, and reduced emissions. Updates to reactor design and selective catalysts steer the process away from toxic intermediates. Some researchers experiment with using captured carbon dioxide as a feedstock, closing the carbon loop and boosting the “green chemistry” profile for synthetic routes. In agriculture, new mixtures with other acids or fermentation byproducts aim to keep animal gut health in check while reducing antibiotic resistance, a key battleground in the wider fight against superbugs.
Toxicity Research
Basic studies confirm that formic acid packs a punch even at low exposure: skin contact leads to painful blisters, and inhaling fumes can burn respiratory linings. Chronic exposure links to liver and kidney stress, so industry-backed studies push for better monitoring of air and surface levels. Toxicological data support tough occupational hazard limits; researchers cross-check symptoms in exposed workers, livestock, and environmental release sites. Animal models mimic field settings to understand cumulative effects, and large-scale health reviews help governments fine-tune legal thresholds in fertilizers, feed, and food safety codes.
Future Prospects
Alongside familiar roles in food and textiles, formic acid holds new promise as an energy carrier—scientists explore ways to use it as a safe hydrogen storage liquid for fuel cells. Ongoing innovation in clean manufacturing seeks to make formic acid without fossil feedstocks, using renewable resources and captured CO2. Developments in recycling processes might soon lower energy use, shrinking the industry’s overall environmental footprint. As climate pressures grow, formic acid’s old uses in agriculture and preservation become more important, but its future rests on smart investment in safer handling, cleaner synthesis, and versatile end uses, echoing bigger trends across chemistry and manufacturing.
Old Chemical, Modern Jobs
Take a walk on a farm, and the smell of silage may hit you. That sharp scent, underneath the grass and corn, often comes from formic acid. For years, farmers have turned to this simple molecule to keep animal feed fresher, longer. It controls mold and stops bacteria from ruining months of hard work and big investment. Wet silage or hay can spoil in days, costing farmers not just cash, but also time and peace of mind during tough seasons.
From Anthills to Industry Floors
Long ago, scientists pulled formic acid out of ants—literally, from their bodies. Today, vast factories make it in bulk. Chemists pour it into more products than most people realize. If you read the label on your cleaning fluid at home, you might see it in the list. It works against limescale and keeps things fresh in a way gentler than many harsh chemicals. Factories in the leather and textile business soak hides in formic acid to tan or dye them. Without it, leather loses toughness and color doesn’t last. Textile workers rely on it to set color dyes so shirts and pants don’t bleed in the wash.
Food and Feed: Life’s Essentials
Formic acid’s place in the feed industry stretches even further than silage. In many countries, millers use it to keep grain mold-free during storage. That helps fight food loss, a problem that costs billions and leads to food shortages every year. Look at countries with damp climates; bread can become scarce if grain spoils after harvest. Formic acid steps in quietly to hold off hungry fungus and bacteria.
A Tool Against Disease
It may come as a surprise, but beekeepers reach for formic acid to fight parasites, especially the destructive varroa mite. Healthy bees mean better pollination across fields—apples, almonds, blueberries. All depend on strong bee colonies. In my own backyard, I watched a local beekeeper save his hives by vaporizing formic acid to kill mites. He kept his harvest strong that year, and local fruit growers saw a good blossom come spring.
Industrial Power and Environmental Choices
Many industries, from rubber to pharmaceuticals, see real benefits in formic acid because it handles reactions and neutralizes waste. It acts as a reducing agent, nudging chemical processes in the right direction. The trend now is to look for greener, easier-to-handle chemicals. With a smaller environmental footprint than many alternatives, including strong mineral acids, formic acid gets extra attention. In some cases, wastewater treatment plants dose it to help remove heavy metals.
Safety and Smart Handling
Of course, formic acid has its dangers. The fumes sting the nose, just as ants defend their nests with a quick burst of it. Workers around concentrated formic acid need goggles, gloves, and careful training. Keeping containers labeled and out of reach prevents accidents, especially in crowded factories.
Paving a Smarter Path Forward
Looking ahead, the chemical industry aims to use less resource-heavy production and offer safer packaging. For feed and farming, work continues on better delivery systems, so formic acid does its job with less waste. Regulators and researchers keep close track of how it’s used—testing for residues in food, checking safety for animals and workers, staying watchful for better solutions.
Trust in chemistry grows out of real results. Whether preserving feed, strengthening leather, or fighting pests in hives, formic acid proves its usefulness day after day. Knowledge shared between scientists and those who use it keeps the risks in check and lets this old chemical find new roles in a changing world.Everyday Encounters and Exposure
Formic acid shows up in more places than most folks realize. Found in the stings of ants and in some foods, this chemical takes its name from the Latin word for ant. On a farm, it sometimes pops up in bale preservatives. At factories, it helps in leather tanning and rubber production. While it sounds plenty technical, formic acid isn’t rare. That’s where the story gets complicated: just because something appears in nature doesn’t mean it’s always safe in larger amounts.
How Formic Acid Affects the Body
In small doses, formic acid doesn’t usually cause big problems. Touching an ant, brushing against a stinging nettle, or cleaning up after a bug bite won’t put most people in danger. Trouble often shows up with concentrated forms. On the skin, formic acid burns. I remember helping a handyman repair a pump used for mixing chemicals on a farm. A drop of formic acid landed on my arm and left a painful red patch that lasted days, far worse than a mosquito bite. Eyes are even more delicate—a splash can damage vision.
Breathing in formic acid vapors hurts the throat and lungs. Factory workers sometimes develop chronic coughs or start feeling short of breath, especially in places lacking good ventilation. Over time, repeated exposure may inflame the airways or hurt lung tissue. Ingesting large amounts can damage the kidneys or nervous system and bring on nausea, vomiting, and possibly confusion. While such cases are rare, accidents happen where people mistake industrial chemicals for water, leading to real harm.
Stories from the Field
In agriculture, formic acid gets added to animal feed and hay storage to slow spoilage. Farmers know not to handle it with bare hands. Wearing gloves and keeping a steady airflow in the work area makes a real difference. I learned from a fourth-generation dairy farmer who sees more value in caution than heroics. He keeps safety goggles and chemical-resistant gear within reach. He once had a hired hand faint from inhaling vapors, needing a quick trip to the hospital. That day, no one questioned the need for protective habits.
In labs and manufacturing, experienced staff train newcomers on the right way to handle formic acid. Accidents often happen with new workers who rush or skip steps. In my early days working as a lab technician, the supervisor drilled us on keeping containers closed, labeling everything, and never working alone around strong acids. Mistakes carry big consequences—as anyone who has dealt with a skin burn or eye injury can confirm.
Building Safer Spaces
The Occupational Safety and Health Administration (OSHA) sets limits on how much formic acid workers can safely breathe—5 parts per million as an eight-hour average. Anyone using it in bulk needs training, not just a warning label. Simple steps help: wear gloves, goggles, and chemical aprons. Stores should keep acid locked away from casual traffic, never near food or drink areas. Good ventilation systems pull vapors away from breathing zones. In places where kids might poke around, chemicals stay out of reach.
At home, most folks won’t bump into concentrated formic acid unless working on a special project or buying industrial supplies. Even then, it’s worth reading the safety sheets and following the same common-sense rules used on farms and in labs.
Takeaway on Formic Acid and Health
Formic acid works as a valuable tool in agriculture and industry but calls for respect. In my experience, direct contact or careless handling causes trouble, not casual encounters in daily life. Just like fire or power tools, it’s all about knowing the risks and sticking to smart habits. With the right approach, people stay safe, and work gets done.
The Real Impact of Concentration
Most people see formic acid as another industrial chemical. For folks in farming, textiles, leather, and even beekeeping, the quality of formic acid can make or break a process. When I worked with an agricultural supplier, we paid close attention to the concentration. Farmers trusted us to provide something in the 85% to 90% range for silage and pest control, pretty close to pure but not so strong it would damage skin or equipment.
The label might read “98%” or “tech-grade,” but that last 2% can hide real dangers. Water is usually the main impurity, yet even a little extra water changes how formic acid behaves. You need the right strength for the job. Too much water and it won’t preserve fodder or kill mites the way it should. I once saw a shipment mislabeled at 92%, which led to a drop in forage quality on a dairy farm. Cows got sick, and the client lost trust in the supplier. Accurate concentration matters, not just for profit but for safety and animal health.
Pride in Purity
Purity goes deeper than a single percentage. High-purity formic acid — usually above 99% — keeps industries running smoothly. The few impurities left after distillation can mean leftover methanol, iron, or even traces of sulfuric acid. Working at a textile finishing plant, I remember downtime caused by iron contamination in a supposedly pure drum. Fabrics showed unexpected discoloration, which led to returns and profit loss. Tracing the source, we found just a tiny impurity spoiled an entire batch of cloth.
Analytical labs lean on true high-purity acid when running sensitive tests. In the food industry, those same impurities can mean failed audits or even recalls. Safety data from regulatory agencies show that even 0.5% impurity can introduce side products that compromise the outcome. In my experience, the best suppliers readily share certificates of analysis and explain how they keep purity above 99%.
Why the Source and Verification Count
Where your formic acid comes from affects everything down to shelf life. I learned this lesson the hard way buying in bulk from a new overseas producer for cost savings. The acid arrived slightly yellow with cloudiness at the bottom. That’s a warning sign: possibly metallic ions, organic debris, or breakdown products. We tested it with simple lab titration and found only 87% concentration instead of the promised 98%. Rejecting the whole batch cost extra time and cash. My advice: never skip independent testing, and always demand clear sourcing.
Better Handling, Fewer Surprises
Formic acid draws water from the air, so even great acid will slowly drift down in concentration if stored in a humid warehouse. Good producers use airtight containers and train warehouse staff. During a summer heatwave, poorly sealed drums turned to weak acid before they even left the dock. Smart companies invest in climate control and monitor inventory, which saves plenty of headaches down the road.
Solutions That Build Trust
Customers want formic acid that meets their spec every time. Clear data sheets and third-party testing settle nerves fast. Reliable trade partners build their reputation batch by batch. I’ve worked with suppliers who ran spot checks, compared acid to industry standards, and showed lab results before shipping. That transparency isn’t just about compliance — it’s part of respect. Proper education for end-users helps, too. Knowing what to look for on a label, how to spot off-color or cloudy liquids, and learning simple titration in the field all raise quality up and keep accidents down. Quality in, quality out; the details really do matter.
A Closer Look at Why Formic Acid Demands Respect
Anyone who’s spent time near a chemical storage area or worked in food processing or agriculture will tell you: formic acid requires extra care. This sharp-smelling liquid doesn’t play around with skin, eyes, or materials. Slip-ups hurt, both literally and financially. The point here is to keep things simple, safe, and smart—because it’s not just about following regulations. It’s about people, places, and the lingering impact of mistakes.
Understanding the Risks Up Close
One drop splashing on bare skin triggers a burning sensation most won’t forget. Inhalation or direct eye exposure can cause far more serious problems. I remember my first lesson in a chemical lab: folks who had gotten even a trace on their gloves had to stop everything, wash it off, and inspect for spots, because even a few seconds started a reaction. Over time, improperly stored formic acid can eat through metal drums, carve holes in shelves, or seep into storage rooms. That’s not some distant tale—it happens in small shops and big warehouses alike. One small unnoticed leak can lead to thousands in cleanup and medical bills, or even worse, permanent harm.
Safe Storage: Learning from Actual Incidents
Formic acid vaporizes easily, turning into a mist that chills your nose and throat. Letting containers stand open, or storing them somewhere you forget about, sets up a chain reaction that’s hard to break. People end up with headaches, sore lungs, or burned throats. You want plastic or stainless steel containers, tightly sealed, clearly labeled, away from heat sources and strong oxidizers. Stories pop up where workers thought, “Just for now, I’ll put it here”—and then everyone scrambled to fix the damage.
Chemical-resistant trays and proper shelving help avoid drips collecting inside cabinets. Floor-level storage leaves drums vulnerable to forklifts and boot-kicks. Elevated racks with secure supports stop both impact and casual tampering. I’ve seen service calls where acid scored the insides of a cinderblock wall because a wooden pallet collapsed, releasing a barrel. Failures start small; the aftermath escalates fast.
Handling Practices Worth the Effort
Handling doesn’t begin with pouring or measuring. Training sets the stage. If you haven’t walked through a spill response drill or seen a demonstration, you’re almost guaranteed to freeze in an emergency. Fast reaction with plenty of running water offsets a lot of pain. Full goggles and acid-proof gloves stop nearly every routine accident.
Fill or decant in well-ventilated areas, far from traffic routes. Always double-check the identity on the label. Mixing it with bleach, hydrogen peroxide, or other acids—even a splash—kicks up dangerous fumes. I’ve watched seasoned workers find out the hard way after reusing old bottles. Educating new team members and running repeated safety checks brings down the number of emergencies. Each year, OSHA reports include injuries that would vanish if everyone followed these basic, real-world habits.
Common Sense Solutions Work Best
Formic acid management doesn’t demand the latest sensor or a lab technician in a white coat overseeing every move. It calls for real-world attention, periodic inspections, and a willingness to spend a bit more on safe materials. Routine reminders—posters on the wall, toolbox meetings, safety updates—reinforce the message. Prompt cleanup, open communication, and visible records send a stronger signal than any regulation. Costs drop, safety satisfaction rises, and you gain peace of mind knowing the next person who handles that bottle will get home safe.
Understanding Formic Acid’s Role
Formic acid might not get headlines like manufacturing giants or breakthroughs in medicine, but it quietly supports industries from agriculture to cleaning products. It’s the ingredient behind silage preservation on farms and plays a critical role in leather tanning. Even in electronics manufacturing, workers rely on its properties. Moving even a small amount of this chemical across borders raises hard questions about safety, regulation, and transparency.
The Regulatory Hurdle
Most people won’t realize that formic acid, though naturally found in ant venom, counts as a hazardous material under most shipping laws. It causes burns, irritates skin and eyes, and can be toxic if mishandled. Companies can’t tuck it into a box, slap on a stamp, and send it to another country.
Every country takes a slightly different approach. The United States, for example, holds importers to strict rules under the Department of Transportation and Environmental Protection Agency. The European Union sets its own measures under REACH, demanding detailed documentation and clear hazard labeling.
Every shipment attracts paperwork, from Material Safety Data Sheets to proof of correct packaging. Carriers like DHL and FedEx often list extra requirements and may turn away shippers who miss a single step. Even experienced exporters sometimes get caught out by an outdated certificate or changes in classification.
Why Controls Matter
I spent time working in a metals processing plant, where every drum of formic acid needed to be accounted for. Even routine deliveries called for careful planning, because an accident could put workers and neighbors at risk. On top of direct dangers to people, a container spill could damage equipment and trigger expensive cleanup efforts.
Global trade depends on consistency and trust, so ignoring these controls can grind businesses to a halt. Countries who receive poorly labeled dangerous goods risk environmental and public health disasters. Legitimate users end up waiting for reships, with production lines or agricultural cycles suffering delays.
Smaller manufacturers often bear the brunt of these challenges. Large corporations usually have compliance teams and systems in place. Familiar with startup scenes, I’ve watched new entrepreneurs struggle to figure out how to move products safely—and sometimes abandon perfectly good ideas, not for lack of market but for lack of regulatory clarity.
Finding A Path Forward
Modern supply chains run on information. Traceability, up-to-date SDS documents, digital declarations, and verified packaging methods would lessen headaches across industries. The chemical sector could benefit from international certification standards and one-stop portals that demystify changing rules—and automate filing wherever possible.
Expansive partnerships between regulators and industry associations have already begun easing the way. Some regions have piloted end-to-end digital shipment tracking, cutting down on errors and improving emergency response. Encouraging wider adoption of these methods would reduce risk for both people and the environment.
Safe shipping isn’t just about following rules on paper. It draws on shared responsibility, technical knowledge, and a commitment to public health. Trust grows when everyone believes the person sending the formic acid takes every precaution, not because they fear a fine, but because lives may depend on it.
| Names | |
| Preferred IUPAC name | Methanoic acid |
| Other names |
Methanoic acid
Hydrogen carboxylic acid Aminic acid Formylic acid |
| Pronunciation | /ˈfɔːrmɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 64-18-6 |
| Beilstein Reference | Beilstein Reference: 1718733 |
| ChEBI | CHEBI:28473 |
| ChEMBL | CHEMBL506 |
| ChemSpider | 454 |
| DrugBank | DB01942 |
| ECHA InfoCard | 03bfa209-7d63-407c-95a1-c4ceb2ab0e6a |
| EC Number | 200-579-1 |
| Gmelin Reference | 209 |
| KEGG | C00047 |
| MeSH | D006707 |
| PubChem CID | 718 |
| RTECS number | LR0350000 |
| UNII | 64XW99BT4I |
| UN number | UN1779 |
| Properties | |
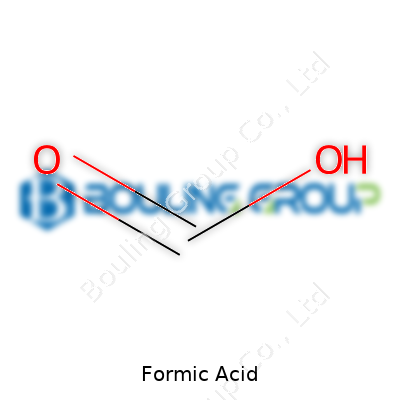
| Chemical formula | HCOOH |
| Molar mass | 46.03 g/mol |
| Appearance | Clear, colorless, fuming liquid with a pungent, penetrating odor |
| Odor | Pungent, penetrating |
| Density | 1.22 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.54 |
| Vapor pressure | 5.7 kPa (at 20 °C) |
| Acidity (pKa) | 3.75 |
| Basicity (pKb) | 10.33 |
| Magnetic susceptibility (χ) | χ = -21.3×10⁻⁶ |
| Refractive index (nD) | 1.371 |
| Viscosity | Viscosity: 1.57 mPa·s (at 20 °C) |
| Dipole moment | 1.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 130.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −425.0 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | −254.0 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | J01XX01 |
| Hazards | |
| GHS labelling | Warning. Flam. Liq. 3, Acute Tox. 3, Skin Corr. 1A, Eye Dam. 1 (H226, H301, H311, H314, H318) |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H226, H314, H331 |
| Precautionary statements | P280, P261, P264, P271, P303+P361+P353, P305+P351+P338, P304+P340, P312, P330, P337+P313, P363, P501 |
| NFPA 704 (fire diamond) | 3-2-2-A |
| Flash point | approximately 69°C |
| Autoignition temperature | 601 °C (1114 °F; 874 K) |
| Explosive limits | 3.7–33% |
| Lethal dose or concentration | LD50 oral rat 730 mg/kg |
| LD50 (median dose) | LD50 (median dose) of formic acid: 730 mg/kg (oral, rat) |
| NIOSH | B0268 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Formic Acid: "5 ppm (9 mg/m³) as an 8-hour TWA (OSHA) |
| REL (Recommended) | 30 mg/m³ |
| IDLH (Immediate danger) | 30 ppm |
| Related compounds | |
| Related compounds |
Acetic acid
Methanol Formaldehyde Oxalic acid Sodium formate Methyl formate Carbon monoxide |

