Understanding Isophorone: A Comprehensive Commentary
Historical Development
Back in the early 20th century, as chemists searched for new industrial solvents and specialty chemicals, isophorone emerged from cyclization reactions designed to make use of byproducts in acetone processing. Industry pioneers like Shell and Bayer invested in scaling up its production during the 1940s and 1950s, recognizing its unique structure could open new doors in coatings and polymers. As paint and plastic manufacturers looked for more robust solvents and monomers, isophorone steadily found its niche. Patents in the 1960s solidified best practices for purification, influencing modern global standards for intermediates in high-performance materials.
Product Overview
Isophorone is an organic compound used mainly as a solvent and building block in synthesis. Modern manufacturing uses it to support everything from protective coatings to specialty adhesives. Suppliers usually offer it in large drums or ISO containers, ready for key industrial processes. Companies use its unique liquid state and moderate evaporation rate to balance performance in inks and coatings where other solvents fall short. Its slightly peppery, camphor-like odor signals its presence, a reminder of its significance in many chemical formulations.
Physical & Chemical Properties
This colorless liquid stands out for its high boiling point around 215°C, making it useful for applications where heat-stable solvents keep production stable. Its molecular structure (C9H14O), with a six-membered ring and unsaturated bonds, allows it to perform unique chemical tricks. The density sits close to 0.92 g/cm3 at room temperature, and it shows moderate solubility in water, excellent miscibility with organic liquids, and notable resistance to spontaneous degradation. Such a balance enables formulators to use isophorone where slow drying and good solvent power are required for polymers, resins, and specialty inks.
Technical Specifications & Labeling
Industrial suppliers offer isophorone with purity typically exceeding 98%, minimizing byproducts like diisophorone or high-boiling aldehydes. Labels for shipping and handling carry UN number 1915, and technical data sheets spell out physical constants, impurity limits, recommended handling conditions, and shelf life expectations. Industries value the clarity of information on flash point (about 85°C), vapor pressure, and chemical compatibility, often provided in comprehensive safety data sheets, supporting both compliance and process safety.
Preparation Method
Large-scale isophorone production involves acetone self-condensation under alkaline conditions. In practice, manufacturers run a catalytic cycle, feeding acetone and recovering isophorone as a distillate. Sodium hydroxide or barium hydroxide acts as the catalyst to push conversion forward. Unreacted acetone gets recycled, which helps minimize waste and save on costs. Vacuum distillation polishes the final product, removing remaining water or volatile byproducts, ensuring downstream users have a consistent, high-purity solvent for sensitive reactions.
Chemical Reactions & Modifications
Isophorone has a highly reactive structure making it a key intermediate in many chemical transformations. Addition across the double bond allows for hydrogenation, turning isophorone into isophorone dihydro or isophorone diamine, each unlocking further end-use opportunities. Its carbonyl group enables classic reactions with hydrazine, Grignard reagents, or reduction agents, vital in making specialty chemicals like amines and polyfunctional resins. Its presence often signals the fine balance between solvent activity and stability—a feature valued by synthetic chemists and formulators.
Synonyms & Product Names
Chemists across the globe recognize isophorone under alternate names such as 3,5,5-trimethyl-2-cyclohexen-1-one or just IP. Drums may show commercial brands like VESTASOL IP or Clearphorone, alongside its CAS number 78-59-1. These aliases reflect decades of commercial and academic work, smoothing the way for cross-border shipments and regulatory clearances.
Safety & Operational Standards
Safe use of isophorone requires attention due to its toxicity and volatility. Prolonged inhalation or skin contact can harm liver and kidney function, as concluded by toxicology trials and exposure studies. Occupational standards, such as an OSHA permissible exposure limit of 4 ppm, guide facility design and PPE protocols. Proper ventilation, fire suppression systems, and spill containment work together to meet both legal mandates and practical safety goals on the shop floor. Labeling standards conform to GHS guidelines, with clear warnings about flammability and chronic health risks.
Application Area
Paint formulators depend on isophorone for smoothing polyurethane coatings and improving wetting in waterborne systems. Inks and adhesives benefit from its moderate evaporation rate, allowing for workable open times and superior finish quality. Epoxy specialists convert isophorone into diamine curing agents, making tough, chemical-resistant pipelines and tanks. Beyond coatings, it finds use in pesticides and herbicide synthesis, exploiting its solvency and ease of chemical modification. Floor finishes, varnishes, waterproofing materials, and some engineered plastics incorporate isophorone for robust performance under chemical and physical stress.
Research & Development
Academic teams and industrial R&D units keep examining isophorone’s role in new polymers, specialty surfactants, and pharmaceutical intermediates. Recent research includes greener production methods, using recyclable or biocatalysts and tighter reaction controls to save resources. Teams also look at copolymer systems incorporating isophorone, aiming to push boundaries in flexible electronics and advanced adhesives. Conferences dedicate sessions to isophorone as both a legacy chemical and an expandable platform for novel materials.
Toxicity Research
The U.S. Environmental Protection Agency and European regulators cite repeated animal studies showing reproductive toxicity and organ damage at high doses. Long-term inhalation studies pinpoint possible carcinogenic risks, mandating strict monitoring. Workplace studies highlight acute effects—dizziness, headache, and skin irritation—reinforcing the call for enclosed handling and medical checkups. Data gaps remain in low-dose chronic exposure, so researchers keep pushing for updated epidemiological studies and detailed mechanistic research.
Future Prospects
Growing demand for eco-friendly solvents drives new research into bio-based isophorone made from renewable acetone. Regulatory pressure on volatile organic compounds motivates the search for lower-emission processes and closed-loop systems for both production and end use. New end-markets—like specialty polymers, resistant coatings for electronics, and low-toxicity agrochemical intermediates—promise to lift demand. Ongoing investment from both established chemical giants and green chemistry startups reinvigorates isophorone’s standing as a critical building block for next-generation materials and sustainable manufacturing.
A Key Ingredient Far Beyond the Lab
Isophorone might sound unfamiliar, but it shows up in plenty of everyday situations. You’ll find it acting in heavy-duty paints, varnishes, and even some cleaning solutions. I used to work near a large industrial coatings facility, and it surprised me to learn how one chemical helped keep factory floors gleaming and bridges from rusting out in the next big rain. People walking by never give that effort a second thought, but it’s clear that isophorone has staying power in manufacturing for a reason.
How Industry Relies on It
The power of isophorone comes from its role as a solvent. In paint and coatings, it lets pigments mix smoothly, helps the paint flow evenly, and slows down evaporation just enough to avoid bubbling and streaks. Industrial-strength cleaners also benefit from its unique properties, breaking down tough residues that water alone can’t touch. When I painted old farm equipment years ago, I noticed how solvent-based paints dressed the metal in a coat that weather couldn't strip away, even after seasons outside.
Printing inks draw on isophorone for a similar reason—colors show up crisp and dry without clogging up the machinery. Leather workers, too, lean on isophorone in adhesives and finishes. Anyone owning an old leather jacket has probably, unknowingly, seen its effects in action.
The Reality of Health and Environmental Concerns
Working around isophorone isn’t risk-free. The smell hits hard, even with a mask, and prolonged skin contact or breathing high levels brings irritation and potential health problems. Science backs up these risks. Chronic exposure has connected to headaches, nausea, and even long-term organ issues. Communities living close to manufacturing plants keep raising concerns about chemical leaks and air quality, which hit home for me when I once lived downwind from such a site. Those stories from neighbors about headaches and unexplained smells never left my mind.
Environmental effects can snowball, too. Isophorone doesn’t break down overnight in rivers or soil. Fish and other wildlife end up at risk after nearby spills. Scientists have flagged its impact on aquatic life, and communities have pushed for tighter monitoring and better leak prevention.
Why Vigilance Beats Complacency
Isophorone keeps industry running, but regulations matter. Factories with strong safety cultures install proper ventilation, provide workers with solid protective gear, and invest in leak prevention. Government agencies keep checking air and water for unsafe levels, pushing for regular inspections and transparent reporting. Where I’ve seen success, companies worked directly with local communities, shared data, and allowed real input on safety plans.
Ultimately, keeping people safe comes down to attention at every step—from the research lab, through production, right to the workers on the floor and the neighborhoods down the street. If shoppers and business owners demand safer products and governments enforce tough standards, together we can limit the downside of a powerful chemical like isophorone, while still benefiting from its strengths.
Understanding Isophorone Risks
Isophorone shows up in a lot of industrial settings, often used as a solvent or in the production of coatings and inks. People working with it quickly notice its strong, sharp smell and how quickly it evaporates. Personal experience teaches you to pay close attention to your surroundings—breathing in even low concentrations over time can bring headaches, dizziness, or even more serious long-term problems. Some studies, like those referenced by the US National Library of Medicine, connect isophorone exposure to liver and kidney effects when safety practices go ignored. The liquid itself irritates skin and eyes. It takes discipline and the right gear to keep things safe.
Personal Protective Equipment
Wearing gloves made of nitrile or neoprene keeps this chemical from soaking into your skin. Thin rubber gloves break down, quickly turning into a risk instead of a help. For jobs that might splash, face shields or goggles give real peace of mind—eye contact leads to immediate, painful stinging and potential long-term harm. Coveralls and long sleeves matter because isophorone vaporizes and can cling to fabric. I’ve seen plenty of coworkers brush caution aside, only to develop rashes or breathing trouble by the end of a shift.
Ventilation and Air Quality
Nobody appreciates the subtle danger in poor ventilation until dizziness creeps up halfway through a task. Isophorone evaporates fast. Good airflow, whether through local exhaust fans or open-air work spaces, reduces vapor buildup. Some go the extra mile by using air monitoring devices right near the workspace. NIOSH provides clear guidelines, encouraging regular checks if work ramps up or shifts last longer. Even simple steps make a difference—in my own workplace, propping open doors and windows lowered the chemical odor, and headaches and complaints died down.
Spill Response and Hygiene
Spills happen quickly, and every worker should know what to do, not just supervisors. Quick containment works best: absorbent pads and sand tackle small spills, but bigger accidents demand trained response. Washing up before lunch might feel like a hassle, but skipping it raises the chance of chemicals finding their way into food or onto your face. Soap and water after handling is a non-negotiable routine. In my early days on the job, I ignored this advice once and tasted the chemical for hours afterward—a lesson I never forgot.
Safe Storage and Disposal
Fire risk grows if isophorone sits in open or poorly sealed containers. The flash point sits dangerously low, so closed, clearly-labeled containers prevent accidental ignition. Storing away from sparks, flames, and incompatible substances cuts back on potential disasters. Facilities keep disposal logs and work with licensed chemical waste handlers, since pouring leftover isophorone down the drain invites both regulatory trouble and environmental damage.
Training and Awareness
Ongoing training, not just the day you start the job, keeps safety knowledge current as processes or regulations shift. OSHA details are clear about the need for updated records and ongoing hazard communication. If everyone, not just managers, understands safety protocols, quick reactions and fewer incidents follow. Simple, upfront communication helps avoid the confusion that can crop up during busy, high-pressure jobs.
Looking Forward
Keeping people and the environment safe around isophorone takes both personal responsibility and strong workplace policy. By focusing on real protection—proper gloves, solid ventilation, fast cleanup, responsible storage, and regular training—we sidestep most of the dangers and set a foundation for healthier workplaces.
The Simple Facts About Isophorone
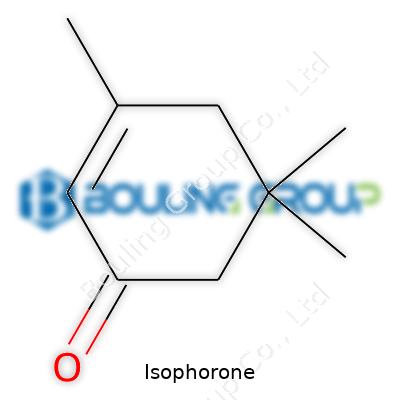
Isophorone, with the chemical formula C9H14O, might not sound familiar outside certain factories or laboratories, but it shows up more often than you might think. Chemically, this liquid stands as a colorless to pale yellow compound, slipping into stronger industrial solvents, coatings, and even pesticides.
The structure draws extra attention among chemists: a six-membered cyclohexene ring dressed up with a methyl group and an isopropylidene group. The molecule looks like this: 3,5,5-trimethyl-2-cyclohexen-1-one. That’s a mouthful, yet break it down and you find three methyl groups hanging onto a cyclohexene ring with a lone oxygen in a ketone group. This combination gives isophorone properties you just can't find in simpler solvents.
Real-World Importance
Factories rely on isophorone because water, paint, and a few other chemicals aren't so compatible. I remember, back in the day, working with leather finishing — the best blends usually needed a dilution step, and isophorone never let me down. It dissolves those stubborn resins and dyes that water turns its back on. Paints and varnishes come out smoother. Pharmaceutical labs trust it to pull out certain active ingredients that would otherwise stay stuck. So it's more than a lab curiosity; it's a workhorse.
Digging through numbers, the EPA and European Chemicals Agency both note its place as a high-production-volume chemical. A global market review reported annual consumption in the range of tens of thousands of tons, echoing just how much demand rides on its unique chemistry. Its low evaporation rate and ability to mix with polar and nonpolar chemicals set it apart from weaker agents like acetone or ethyl acetate.
Concerns Alongside Convenience
Widespread doesn't mean worry-free. Exposure to isophorone can irritate the nose and skin, and, after long-term contact, even trigger liver and kidney issues according to occupational safety research. This isn't the kind of solvent to splash around in open buckets. Most regional regulators, including OSHA and the European Chemicals Agency, draw firm lines on exposure limits. Cleanroom experiences taught me pretty quickly the value of proper gloves and good ventilation whenever we pulled out the isophorone drum.
There’s another angle: the environment takes a hit if disposal isn’t controlled. Isophorone floats along in waterways and doesn’t break down easily, which gives rise to concern about aquatic toxicity. Studies from the US Geological Survey point out that it sticks around for days, if not weeks, in rivers.
Better Practices and Safer Alternatives
Switching to safer chemicals wherever possible makes sense. Some coatings companies have started to drop isophorone in favor of newer, lower-toxicity solvents derived from green chemistry, such as lactate esters or certain glycols. Swapping out old school for new takes upfront investment, but the payoff comes through reduced hazards for workers and fewer headaches about downstream pollution.
It’s easy to overlook small lab safety choices. Simple fixes — local exhaust fans, chemical-resistant gloves, labeled containers — save a lot of trouble. Plant engineers can trim exposure by using closed-loop systems and on-site solvent recycling. In a tight regulatory environment, these shifts not only keep the crew healthy but also keep the operation running without compliance stumbles.
So yes, isophorone remains popular, and its chemical quirks still solve problems off the shelf. At the same time, careful stewardship, training, and gradual upgrades toward safer substitutes create a win-win, both for workers’ health and for the land and water everyone counts on.
What Is Isophorone?
A lot of paints, inks, and adhesives wouldn’t work the same without isophorone. This solvent flows into manufacturing spaces because it dissolves tough substances and gives coatings that extra bit of flexibility. Factories value it for these jobs, but it doesn’t come without downsides.
The Health Side: Breathing and Touching Concerns
If you’ve ever worked around strong-smelling chemicals in an old print shop or paint booth, you know the worry that sticks with fumes. Isophorone vapor fills the air fast in an enclosed space. People who breathe it in can start to cough and feel dizzy. The stuff has a sharp smell that often signals trouble. Longer exposure has a record of causing headaches, nausea, and sometimes even discomfort in the chest. On rare occasions, workers have reported liver or kidney problems after a long history around it.
Touch is another concern. Some folks get a rash if isophorone lingers on their hands. The chemical penetrates skin more easily than a lot of people expect. It creeps into cuts but also seeps through gloves if they aren’t chosen with care. Eye irritation comes fast too, just from vapors, giving that strong stinging burn that says you need fresh air.
Studies done by the EPA link isophorone to possible cancer risk when people face it often enough. These findings prompted tighter safety rules for workers and call for careful handling at home or in small businesses.
What It Does to Water and Soil
Isophorone doesn’t just disappear after leaving the factory. Spills rinse into storm drains and rivers after heavy rain. Once in the water, fish and bugs pick it up. The substance breaks down pretty slowly, hanging around longer than some other solvents. Environmental experts found that certain aquatic life suffers from exposure at low doses—fish lose their balance and can even die off when concentrations spike in warmer months.
Soil isn’t much safer. Even a small leak from a container in a warehouse can spread through the ground. Microbes struggle to break it down quickly, so seeds and roots struggle if the spill is big enough.
Where Responsibility and Solutions Come In
My own time in an auto body shop taught me hard lessons about venting out fumes and keeping storage tight. Too many businesses delay upgrades because good ventilation and spill kits cost money. But the price of inaction can show up in medical bills or future site cleanups.
Simple steps solve big chunks of the problem. Switching out older solvents for safer alternatives reduces risk right away. Workers need more than quick training videos—they need real access to gloves that won’t let chemicals slip through, along with good exhaust fans. Regular checks of pipes and tanks stop leaks before the next thunderstorm carries them away.
Laws now require truth in safety data sheets, so folks mixing paints or printing signs know what they’re handling. Neighbors living near manufacturing plants have begun to push for regular air and water quality testing. Countless towns learned the hard way that prevention saves much more than any cleanup after the fact.
Facts Point to Action, Not Panic
People deserve honest information about the risks that isophorone brings. Science and lived experience point to real hazards, not just for workers but for anyone nearby. Safety tools, clear labeling, and a healthy respect for what these substances can do to bodies and backyards deliver better outcomes for everyone involved.
Keeping Isophorone Safe on Site
A lot of folks outside of industry may never have heard of isophorone. Those of us working in paints, coatings, and chemicals understand this solvent gets the job done, but it arrives with some risks. What stands out first is flammability. Isophorone vapor can catch fire quickly, making it more than a paperwork headache—it’s a matter of protecting lives and property. Drums and tanks holding isophorone need tight, non-leaky lids. No smoking signs aren’t window dressing; insurance and fire codes demand real discipline here.
Exposure isn’t just about a big burn, though. Isophorone kicks up fumes that can leave workers dizzy or worse. Anyone handling this stuff needs decent ventilation. The air systems drain vapors away, which spares folks on the floor and keeps workplace air up to code. Personal protective gear—goggles, gloves, sometimes even respirators—cuts the risk of splashes. In my early days on the warehouse floor, sloppy gloves meant strong headaches by lunch. So, gear up every time.
Heat turns risk into reality fast. Isophorone has a flash point sitting just under 85°C (185°F), so a hot day or a shorted heater can spell trouble. Store it cool, ideally below that number, and far from boilers or sunlight. Chemical compatibility charts deserve respect too. Acids and oxidizers don’t mix with isophorone, so putting cans next to bleach or pool chemicals ignores years of hard-earned wisdom.
Getting Isophorone from A to B
Moving isophorone isn’t as easy as rolling out a truck and loading barrels. Every shipping label and manifest tags it as a hazardous good. Federal rules in most countries treat it just like gasoline or acetone. Drivers carry special paperwork, know proper emergency call numbers, and steer clear of open flames or hot cargo.
Before any isophorone hits the highway, barrels must be checked for leaks and approved for hazardous liquids. Companies use containers rated by groups like the UN or DOT, each batch traceable by lot numbers in case of trouble. Trucks often have spill kits—absorbent pads, overpack drums—along for the ride, since road mishaps don’t make exceptions for anyone having a bad day. I’ve seen a ten-minute spill response drill stop what could’ve been a warehouse evacuation, so these kits shouldn’t gather dust.
Solutions: Learning from the Field
Safety managers and logistics teams have tools to make things run smoother. Updated training sticks with people longer than yearly videos. I remember a situation where a driver caught a forgotten vent plug—his sharp eye stopped vapor buildup before anything left the dock. Regular checks, paired with hands-on walkthroughs, cut down on missed steps.
Digital tools now let teams monitor temperature and pressure in real time, sending alerts to cell phones if numbers drift. Old cooling fans don’t compare to automatic alerts. Records from these systems prove useful for insurance claims, audits, and in company safety reviews—facts settle disputes faster than guesswork.
Above all, staying sharp and treating every shipment with attention protects everyone from plant workers to end users. It’s the right way to do business and the right thing for people. That bit of diligence pays off more than any shortcut ever will.
| Names | |
| Preferred IUPAC name | 3,5,5-Trimethyl-2-cyclohexen-1-one |
| Other names |
Isoforone
3,5,5-Trimethyl-2-cyclohexen-1-one Isoacetophorone |
| Pronunciation | /ˌaɪ.səˈfɔːr.oʊn/ |
| Identifiers | |
| CAS Number | 78-59-1 |
| 3D model (JSmol) | Isophorone JSmol 3D model string: `C1CC(CC(C1)=O)C(C)C` |
| Beilstein Reference | 0326052 |
| ChEBI | CHEBI:17549 |
| ChEMBL | CHEMBL14345 |
| ChemSpider | 7199 |
| DrugBank | DB02119 |
| ECHA InfoCard | ECHA InfoCard: 100.003.380 |
| EC Number | 201-126-0 |
| Gmelin Reference | Gmelin Reference: **136008** |
| KEGG | C06531 |
| MeSH | D010090 |
| PubChem CID | 8499 |
| RTECS number | UF9275000 |
| UNII | Z9G6JI8UX1 |
| UN number | UN1245 |
| Properties | |
| Chemical formula | C9H14O |
| Molar mass | 138.21 g/mol |
| Appearance | Colorless liquid with peppermint-like odor |
| Odor | Peppermint-like |
| Density | 0.92 g/cm³ |
| Solubility in water | 14 g/L (20 °C) |
| log P | 1.7 |
| Vapor pressure | 0.4 mmHg (20°C) |
| Acidity (pKa) | 19.3 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | -7.72×10⁻⁶ |
| Refractive index (nD) | 1.474 |
| Viscosity | 2.57 mPa·s (25 °C) |
| Dipole moment | 2.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 218.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -318.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3660.7 kJ/mol |
| Pharmacology | |
| ATC code | D03AX03 |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS02,GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H332 |
| Precautionary statements | P210, P260, P273, P280, P301+P312, P304+P340, P305+P351+P338, P308+P313, P403+P233, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 85°C (185°F) |
| Autoignition temperature | 485 °C |
| Explosive limits | 1% to 1.2% (lower), 9.7% to 9.8% (upper) |
| Lethal dose or concentration | LD50 oral rat 1,500 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1,900 mg/kg (oral, rat) |
| NIOSH | KGA |
| PEL (Permissible) | 25 ppm |
| REL (Recommended) | 50 ppm |
| IDLH (Immediate danger) | 200 ppm |

