N-(2-Hydroxyethyl)Ethylenediamine: A Closer Look at Its Past, Science, and Applications
Historical Development
Chemists have explored compounds like N-(2-Hydroxyethyl)ethylenediamine for more than a century, mostly because of the drive to improve industrial materials and chemical synthesis. This compound’s story fits right into the evolution of both the chemical industry and life sciences. The early work on alkanolamines, including N-(2-Hydroxyethyl)ethylenediamine, came about as researchers searched for building blocks that combined water solubility with nitrogen’s reactivity. The world tends to notice new chemicals once their usefulness in surfactants, solvents, or chelating agents becomes clear, and that’s how this substance picked up steam. Over the decades, improvements in refining methods and a stronger push for chemical safety kept reshaping the approach, and manufacturers started focusing on consistent purity and labeling.
Product Overview
N-(2-Hydroxyethyl)ethylenediamine, sometimes called 2-(2-aminoethylamino)ethanol, rests among the versatile building blocks for many industrial and pharmaceutical applications. Used in everything from water treatment agents to specialized polymer materials, it offers dual amine and alcohol functionalities, making it useful for linking, buffering, or reacting with a variety of partners. The molecule’s bifunctional nature makes it valuable in the industry as it ties together two worlds: the stability and reactivity of amines and the solubility and hydrophilicity of an alcohol group. This combination keeps it in steady demand, especially in chemical plants and research labs where customization is the name of the game.
Physical & Chemical Properties
The compound appears as a colorless to pale yellow liquid, giving off a slight amine odor typical for molecules in this family. At room temperature, it flows easily, reflecting its moderate viscosity and low melting point. The boiling point reaches well above 200°C, so handling in industrial settings requires proper cooling and fume extraction. Its solubility in water and polar organic solvents is high, helping it blend into a wide array of chemical mixtures. The two amine groups and one hydroxyl group slot into chemical reactions with acids, activated halogens, or isocyanates, and they can accept or donate protons in acid-base chemistry. Its basicity matters in processes where precise pH control or chelation is necessary.
Technical Specifications & Labeling
Chemical suppliers commonly detail purity (often >98%), water content, main impurity profiles, and recommended storage conditions. Proper labeling is more than regulatory red tape; it allows handlers to make informed, safe decisions when integrating the substance into formulations. Bottle labels list not only the product name and batch details but safety warnings, recommended handling temperatures, shelf-life, and compatibility notes with common reagents. Consistent labeling standards keep supply chains transparent and ensure that anyone working with the compound can track every bottle, from manufacture to end use.
Preparation Method
Manufacturing usually follows a straightforward pathway: ethylenediamine reacts with ethylene oxide or ethylene chlorohydrin, forming the hydroxyethyl derivative. The reaction environment must be tightly controlled to limit side products like bis-hydroxyethyl derivatives, as these can dramatically change the profile of the final product. Pressure, temperature, and reaction time are closely adjusted, while modern factories install monitoring to catch unexpected spikes or contaminant formation. Years ago, such synthesis routes used simple batch reactors, but industry has moved toward safer, continuous-flow systems that limit employee exposure and allow for steadier quality.
Chemical Reactions & Modifications
N-(2-Hydroxyethyl)ethylenediamine participates in a large variety of reactions that leverage both its amine and alcohol groups. Alkylation of the nitrogen atoms creates tertiary amine derivatives. Esterification with acids or acyl chlorides alters its water solubility and chemical activity, opening doors to surfactant or polymer chemistry. In my own experience working in synthetic labs, we used this compound as a linker in dendrimer assembly, where its branching points let us grow large, multi-armed polymers for drug delivery research. It stands up well to harsh basic or neutral conditions, but with strong oxidizers or acids, it can degrade or form unwanted by-products, so balancing reactant strength matters in planning out reliable yields.
Synonyms & Product Names
The chemical wears many hats depending on the industry and language. “2-(2-aminoethylamino)ethanol” shows up in European chemical catalogs. North American manufacturers use “N-hydroxyethylethylenediamine” or abbreviate to “HEEDA.” Specialty chemical suppliers often market it under proprietary names, and these sometimes overlap if not managed properly. Each synonym can map to minor variations in purity, intended use, or even batch processing standards, and confusion here risks problems when companies scale up or share recipes across regions.
Safety & Operational Standards
Working with N-(2-Hydroxyethyl)ethylenediamine, gloves and goggles become everyday necessities. Exposure can cause skin or eye irritation, and inhalation at high concentrations may lead to respiratory issues. Industry standards require closed transfer systems and well-ventilated handling areas. Chemical hygiene plans focus on spill control and neutralization protocols, drawing on decades of lessons learned from similar amine alcohols. The push to reduce workplace accidents led regulators to draft tighter guidelines about permissible exposure limits and medical monitoring for frequent handlers. Waste disposal must stick to rules for amines and glycol derivatives since local water tables don’t need more contamination.
Application Area
This molecule finds roles everywhere from industrial water treatment to textile softening to pharmaceutical intermediate synthesis. It crops up in epoxy curing agent blends, providing flexibility or tuning crosslink density in adhesives and paints. Specialists in resin chemistry turn to it when they need fine control over polymer growth rates or branching. Biologists deploy it as a chelating agent for metal ions when prepping samples for analysis. In my experience running custom syntheses for clients, we specified this compound for stabilizing radical polymerizations, and its dual functionality let us click together surprising chemical structures that seemed out of reach with simpler amines. It continues to draw attention in areas where adjustable solubility and basicity can boost process performance.
Research & Development
Scientific teams have invested significant resources in optimizing both the greener synthesis and broader applications of this compound. Enzyme-catalyzed reactions and renewable feedstocks draw funding as companies search for less wasteful, safer pathways to fulfillment. Academic journals publish new ways to anchor therapeutic agents on the molecule’s backbone or to employ it as part of ion-exchange resins. Collaboration between industry and universities tightens the feedback loop, bringing fresh application ideas from the bench to the market. Recent years saw a jump in research output connecting N-(2-Hydroxyethyl)ethylenediamine to high-value pharmaceutical synthesis and the growing demand for custom-tailored water treatment solutions.
Toxicity Research
Amines and alkanolamines carry a reputation for irritancy, and N-(2-Hydroxyethyl)ethylenediamine sits in the low-to-moderate acute toxicity category. Chronic exposure research remains ongoing, and published studies warn about eye and skin irritation, with respiratory effects more likely at high concentrations or poor ventilation. Animal studies show moderate oral LD50 values, pointing to a need for caution but not widespread hazard at typical environmental or occupational levels. Regulatory agencies keep an eye on workplace concentrations, and the chemical safety literature encourages regular reviews of best practices. Product stewardship demands more than following old data; newer research is helping clarify its effects on aquatic species and possible links to chronic endpoints, keeping environmental chemists busy.
Future Prospects
Innovation rarely slows down around such a versatile molecule. Efforts continue to develop less hazardous analogs or process tweaks that cut waste and energy input. The molecule’s dual functionality keeps it in the running for next-generation adhesives, green chemistry formulations, and niche pharmaceuticals. Broader awareness of process safety and environmental footprint will keep shaping how this chemical gets used. From what I’ve seen in trends data and conversations at industry conferences, demand for specialized amine-alcohols like this keeps rising, especially alongside the push for more sustainable water systems and custom material science. As both application and safety research move forward, the sector’s challenge lies in balancing performance, safety, and environmental compatibility—never an easy task but always a worthwhile pursuit.
Understanding Its Everyday Reach
N-(2-Hydroxyethyl)ethylenediamine, or HEEDA as chemists often call it, doesn't exactly roll off the tongue. Still, this compound finds its way into regular routines way more often than many think. In every bottle of shampoo or tube of lotion lining a bathroom shelf, organics like HEEDA quietly help blend things smoothly and make products gentle enough for regular use. My own years working with industrial cleaning companies taught me how much difference one ingredient can make in a recipe—especially when skin contact happens nearly every day.
Where Industry Finds Value
Manufacturers lean on HEEDA as a building block in producing complex chemicals, from detergents to resins. Chemists like to call it a chelating agent, which means it keeps metal ions in check and stops them from gumming up the works—literally. In water treatment plants, this matters, since blocked pipes and corroded machinery cost real time and money. On job sites, I’ve seen pipes that last longer just because folks chose the right blend of helpers interacting down to the molecular level.
Paints and coatings make use of HEEDA too. Paint manufacturers want good laying and stability so jams and clots don’t threaten a whole batch. Adding HEEDA keeps mixtures moving and ensures the cured finish stands up to sunlight, water drips, or the casual “whoops” of a scuffed chair. In the construction world, there’s a real appreciation for products that can hold up across the seasons, and the chemistry working behind the gloss rarely gets enough attention.
Personal Care Products and Consumer Safety
Personal care product formulators look for substances that balance cleansing power with safety. No one wants an ingredient that leaves skin dry, cracked, or red after a scrub. HEEDA acts as a buffer, softening the action of harsher cleaning agents like sodium lauryl sulfate. Whenever I check ingredient lists to avoid irritants (having learned the hard way with sensitive skin), spotting buffering agents like HEEDA makes me breathe a little easier. Such small changes protect everyday users from bigger problems—like chronic hand irritation or flare-ups of eczema.
The Environmental Question
Every ingredient sent down the drain poses an environmental question. Some chemical processes mean byproducts must be handled carefully to avoid harm to soil and water. Responsible companies invest in wastewater treatment, using breakdown processes that catch leftover chelating agents before they reach rivers and lakes. Working alongside environmental scientists, I’ve seen positive change, but the industry keeps facing tough scrutiny. Public trust only grows when manufacturers are transparent and government watchdogs enforce standards.
Room for Improvement
Some researchers are working on finding greener and safer alternatives to even everyday helpers like HEEDA. Biodegradable options are in the pipeline for cleaning and water treatment sectors. Innovation often means upfront investments—but over time they pay off, helping companies stay flexible as regulations change and customer demands shift. In the end, every improvement here makes products a bit safer, homes a bit cleaner, and our water a bit more protected.
Why Awareness Matters In A Chemical Lab
Most folks working around chemicals know this: short lapses open doors to trouble. N-(2-Hydroxyethyl)ethylenediamine looks like a basic ingredient in a lot of processes, whether you’re in pharmaceuticals, rubber manufacturing, or dyes. People use it for chelation, corrosion inhibition, and as a building block for several products. Yet its friendly reputation tends to fade the minute you get it on your skin, or inhale its vapors.
What Exposure Looks Like
A slightly sweet smell doesn’t hint at what it really does. This chemical causes irritation—eyes, lungs, and skin don’t take it well. The moment a drop lands on skin, redness and that burning itch set in. Accidentally swallow even a little, nausea and abdominal pain soon follow. Inhalation isn’t rare during pouring or mixing; when vapors creep up, coughing and throat discomfort become part of the day. Anyone who’s spilled it on a bench knows how quickly symptoms come on, especially without gloves.
Layering Personal Safety
Over the years, the best defense I’ve seen has three layers. Gloves matter—choose nitrile instead of plain latex since it holds up against amines better. Wearing a lab coat stops stains from soaking into everyday clothes and gives another barrier against skin contact. No one looks glamorous in chemical splash goggles, but on busy days these keep eyes safe from unexpected splashes.
Ventilation in the workspace makes a difference you feel in your lungs. A fume hood helps localize fumes, drawing them away before they drift in your breathing zone. I’ve watched too many folks ignore this step and wind up coughing by lunch. If mechanical ventilation isn’t an option, work near open windows or in larger spaces, reducing the strength of airborne molecules.
Housekeeping Still Matters
Spills happen. I’ve cleaned up more than I can count. Absorbent pads or spill kits on hand save time. Once, a simple paper towel spread the liquid thin across a bench and made cleanup even harder. It pays to sweep up broken glass and wash down work areas with plenty of water right away. Engineers and custodians know—residue left behind keeps reacting, sometimes with other common lab chemicals.
Used gloves, contaminated towels, and wash water all need safe disposal. Labeling waste drums keeps accidental mix-ups low. Where I work, we separate amine waste so it doesn’t wind up in the wrong stream and trigger unwanted reactions.
Knowing What To Do In Emergencies
Quick action stops small mistakes from turning big. A dedicated eyewash station somewhere nearby isn’t just about ticking boxes. Whether you’ve taken a splash in the face or feel the sting on your arms, rapid rinsing reduces the damage. I’ve seen cold water flushes halve the recovery time for coworkers caught off guard.
Emergency numbers belong posted on the wall, not hidden in a drawer. People freeze in real moments of panic without step-by-step guides to follow. A laminated chart near the work desk guides everyone, from old hands to students new to the bench.
Improving Safety Culture Day By Day
Without open conversations and routine reminders, even experienced teams let standards slip. At my last workplace, simple weekly check-ins on correct glove use and spill readiness made accidents rare. Fresh eyes catch missing labels or blocked exits. These tools—personal protection, good air flow, spill control, and clear emergency steps—keep labs healthy and predictable. Using facts, vigilance, and care, you protect not just the work, but the workers too.
A Closer Look at the Basics
Sometimes, chemistry likes to throw us a softball. N-(2-Hydroxyethyl)ethylenediamine doesn’t exactly roll off the tongue, but this molecule pulls its weight in research labs and industry. Structurally, it offers a blend of functional groups: two amines and one alcohol. Most chemists learn early that both amines and alcohols usually play nicely with water. I remember mixing ethylenediamine with water in a freshman lab. No swirling or undissolved bits, just a clear solution. That memory sticks out because solubility can make or break your experiment.
Why Water Solubility Changes Everything
Water solubility matters more than most realize. At home, dissolving sugar is simple, but in a lab, getting a compound to dissolve can mean hours saved or lost. N-(2-Hydroxyethyl)ethylenediamine dissolves readily in water for the same reason many simple amines and alcohols do—the molecule forms hydrogen bonds with water. PubChem, a well-respected database funded by the National Institutes of Health (NIH), confirms this, listing the compound as water soluble. Chemical supply companies list it as “freely soluble,” too, which matches what people find in practice. I’ve poured this stuff into beakers myself, and swirling never goes on long. Anyone who works with it will tell you: if you have access to water, you’re good to go.
Applications Count on That Solubility
Here’s where it gets interesting. Flexible solubility opens the door for real-world use. Take pharmaceuticals. Many drugs need water-soluble building blocks for quick delivery inside the body. Chemists often lean on small molecules with both amines and alcohol groups for this reason, tweaking the formula so it’ll dissolve, mix, and reach where it needs to go. N-(2-Hydroxyethyl)ethylenediamine has its roots not just in drug synthesis but across the field—buffer solutions, textile finishing, even as an intermediate for more complicated chemical families. Each of these relies on dissolving it easily and evenly, especially when precision in mixing affects yield, safety, and cost.
Industry Never Forgets Safety
Handling chemicals always brings up one concern: safety. Working with water-soluble compounds like N-(2-Hydroxyethyl)ethylenediamine can make cleanup faster, but it also means skin contact and accidental splashes matter more. The compound may cause skin and eye irritation. The European Chemicals Agency points out that its easy-to-absorb nature requires gloves, goggles, and clear ventilation protocols. Even simple water wash-downs work well for spills, thanks to its ready solubility, so a fast response can limit exposure.
Barriers and How to Break Them Down
Some labs deal with water that isn’t ultra-pure. Minerals and other solutes can mess with dissolution. If mixing feels off, a little warmth or stirring usually sets things right. From experience, tap water often works, but for sensitive reactions, distilled or deionized water prevents side reactions and keeps purity high. This approach helps maintain credibility, especially in regulated industries that need to show clear, repeatable outcomes. Companies like to see certificate documentation, and so do auditors.
Small Changes Ripple in Research
I’ve watched as a simple switch to water-soluble compounds has reshaped process safety and waste handling in my own projects. Less time fighting solubility issues means more focus on solving the big problems we care about. It’s reminders like these that make N-(2-Hydroxyethyl)ethylenediamine’s role in water chemistry matter outside just the textbook. Real progress builds on choices like these, made in the corners of busy labs every day.
Recognizing the Basics
N-(2-Hydroxyethyl)ethylenediamine stands out in the world of organic chemistry for a reason. It’s not something you come across in daily conversations, but among chemists and people who have fiddled around in a lab, its structure sparks curiosity. This compound often pops up in research and manufacturing—things like chelating agents, surfactants, and even some cleaning formulations quietly depend on its quirks.
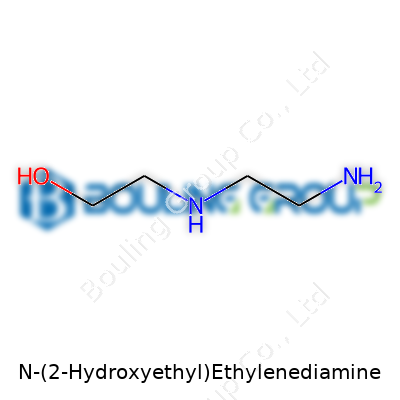
In simple terms, this molecule is built off ethylenediamine, a familiar sight in many chemistry labs. Ethylenediamine itself has two amine groups (-NH2) held together by a two-carbon chain. The "N-(2-hydroxyethyl)" chunk means that one of those nitrogen atoms wears an extra side chain—a hydroxyethyl group, which is just a two-carbon chain with an -OH group dangling at the end.
The Structure—Piece by Piece
Picture the main backbone: NH2-CH2-CH2-NH-. Attach a hydroxyethyl group to one of the nitrogens, and you land at the right architecture for this molecule. That group looks like CH2-CH2-OH, so the full chemical formula comes out as C4H12N2O.
It’s worth pausing to think about what makes this structure matter—each part of the molecule shapes how it behaves. The exposed amine groups lend basicity and allow the compound to bond to metals, while the hydroxy group draws in water and supports hydrogen bonding. That combination brings versatility, useful in chemistry and industry alike.
Real-World Relevance
My own stretch in the lab taught me just how much the smallest addition to a molecular structure changes everything. I once tried making a chelating agent from scratch, and adding a hydroxyethyl group made the new chemical more friendly with water. N-(2-Hydroxyethyl)ethylenediamine behaves in much the same way—a regular ethylenediamine can grab onto metals, but the hydroxy side chain boosts solubility and can change the way other molecules interact with it. Industries don’t chase obscure molecules without reason.
Downstream, this compound often shows up in places you might not inspect very closely. It can serve as a component for liquids that scrub dangerous gases from industrial emissions, a helper in detergents, and even as a building block for more complex chemicals. The structure lets it perform in both water-based and oil-based systems, making it a bit of a chemical chameleon.
Potential Pitfalls and Smarter Choices
Working with compounds like N-(2-Hydroxyethyl)ethylenediamine brings its own set of headaches. Though the molecule looks simple on paper, exposure can irritate skin and eyes, so handling it with gloves and goggles is just routine common sense. Its potential to tie up metals also means careful disposal matters—water treatment plants work overtime to keep such compounds from heading into local rivers where they might skew metal balances for aquatic life.
Better stewardship doesn’t need to wait for new regulations to land. Chemists can sketch out safer alternatives, recycling protocols, or find ways to recover spent reagents. Industries using this compound already tweak reactions to reduce waste and boost yields, which not only saves resources but also lessens the headache later in the process chain.
Looking Forward
Understanding the structure of N-(2-Hydroxyethyl)ethylenediamine isn’t just a box to check for chemistry exams. Appreciation for its design opens the door to innovation—not just swapping parts for novelty’s sake, but creating molecules that work smarter in real-world systems. That’s where future breakthroughs start, and for anyone working with chemistry day in and day out, that edge matters.
Workplace Realities and Basic Precautions
A lot of chemical storage advice sounds robotic until you’ve actually tripped over a leaky drum or had to clean a sticky spill on a hot day. N-(2-Hydroxyethyl)ethylenediamine, usually called HEE or HEEEDA, brings its own set of quirks, especially for people dealing with drums, totes, or lab bottles. It carries a low but stubborn odor and tends to absorb water fast once the seal cracks, making it even slicker in all the wrong places.
Smart storage starts with good ventilation. Fumes rarely announce themselves before headaches hit. A vented cabinet in a cool, well-aired spot cuts down risk right away. Leaving this stuff near workstations only invites skin splashes or accidental mixing.
Forget Fancy Solutions—Basics Win
Keep it locked in a polyethylene or steel drum, with a tight cap at all times, and mark it in plain language. Too many labels fade; printed, laminated hazard warnings avoid mixups. No one wants a “What’s this bucket?” moment during a rushed transfer at closing.
Never line chemicals near potential fuel—oily rags, packaging, even paper towels. HEE isn’t as flammable as some solvents, but high heat breaks it down, creating caustic vapors and turning small mistakes into big messes. If storage means a back shed or warehouse corner, check for sun exposure. Metal drums bake fast by afternoon.
Chilled rooms do more than protect product—they slow down evaporation and cut the risk of decomposition. Keep containers out of reach of doorways, forklifts, and handcarts. People naturally park supplies near shortcuts, yet that’s the route of most awkward bumps and spills.
Human Habits and Emergency Readiness
No matter how many times training covers PPE, it’s easy to skip gloves for “just a small pour.” HEE can irritate skin and eyes enough to cancel a workday or worse. Hazmat supplies—eye wash, spill socks, neutralizers—need to stay closer to the stockpile, not at the other end of the building. People only use what’s nearby in an emergency.
Inspections work better if everyone expects to rotate duties. Fresh eyes spot sticky lids, swelling drums, or water pooling under pallets. Documenting temperature swings, humidity, and leaks isn’t a boring bureaucracy box-tick—it’s the only record if regulators visit or an incident report lands on your desk.
Health, Environment, and Accountability
Regulations won’t always predict your exact workplace, but ignoring safety means ignoring coworkers’ health and the local environment. HEE, if it reaches drains or soils, doesn’t just evaporate—traces hang on and harm aquatic life. A spill kit with absorbent pads next to every chemical zone saves time and complaints from building maintenance or inspectors.
Practical storage keeps accidents from escalating. If your team logs every shipment, dates every drum, and rotates old stock forward, you’ll waste less and dodge crisis calls on weekends. Make one person responsible for weekly checks, and don’t let the routine slip as orders get hectic.
Simple, regular habits—clear labels, cool storage, dry floors, accessible first aid—mean both safety and sanity. In the long run, that earns trust from colleagues, customers, and anyone counting on you to do the job right.
| Names | |
| Preferred IUPAC name | 2-(2-Aminoethylamino)ethan-1-ol |
| Other names |
2-(2-Aminoethylamino)ethanol
Ethanol, 2-(2-aminoethylamino)- N-(2-Hydroxyethyl)ethane-1,2-diamine 2-Hydroxyethyl-1,2-ethanediamine AEEA |
| Pronunciation | /ɛn tuː haɪˈdrɒk.siˌɛθɪl ˌɛθɪˈliːnˌdaɪ.əˌmiːn/ |
| Identifiers | |
| CAS Number | 111-41-1 |
| Beilstein Reference | 1718735 |
| ChEBI | CHEBI:52405 |
| ChEMBL | CHEMBL1230517 |
| ChemSpider | 20239 |
| DrugBank | DB04241 |
| ECHA InfoCard | 07e22e93-9ad7-4d45-aa7a-e807cbb4c48c |
| EC Number | 203-721-0 |
| Gmelin Reference | 137831 |
| KEGG | C06045 |
| MeSH | D08.811.377.500.400 |
| PubChem CID | 12321 |
| RTECS number | KK7175000 |
| UNII | Y9261GRYFY |
| UN number | UN2672 |
| Properties | |
| Chemical formula | C4H12N2O |
| Molar mass | 106.15 g/mol |
| Appearance | Colorless to yellow transparent liquid |
| Odor | Ammonia-like |
| Density | 1.038 g/cm3 |
| Solubility in water | Miscible |
| log P | -2.13 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 9.58 |
| Basicity (pKb) | 5.80 |
| Magnetic susceptibility (χ) | -7.72×10⁻⁴ |
| Refractive index (nD) | 1.482 |
| Viscosity | 13 cP (25 °C) |
| Dipole moment | 2.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 156.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -308.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3433 kJ/mol |
| Pharmacology | |
| ATC code | N07BB03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H332 |
| Precautionary statements | P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 174°C |
| Autoignition temperature | 335°C |
| Lethal dose or concentration | LD50 oral rat 3000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 2,120 mg/kg |
| NIOSH | KW2975000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) for N-(2-Hydroxyethyl)ethylenediamine: "NIOSH REL: 1 ppm (4 mg/m3) TWA |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Ethylenediamine
Diethylenetriamine Triethylenetetramine 1,2-Diaminopropane N,N-Diethylethylenediamine 2-Aminoethanol (Ethanolamine) N-Methylethylenediamine N,N-Dimethylethylenediamine |

