Phenol: Roots, Realities, and the Road Ahead
Historical Background
Chemistry classrooms like to talk about phenol’s arrival as if it lit a spark and changed everything overnight. In truth, phenol’s place in science started over a century ago with coal tar distillation. Friedlieb Runge isolated it from coal tar in the early 1800s and called it "carbolic acid." The backdrop? Crude surgical conditions and overwhelming infections in hospitals. Joseph Lister tested phenol in the 1860s for antiseptic procedures, and, for the first time, wounds healed without festering. More than just a chemical, phenol marked a shift in how people fought disease, powered synthetic dyes, and set the stage for the plastics boom in the 20th century.
Product Snapshot: What Phenol Brings to the Table
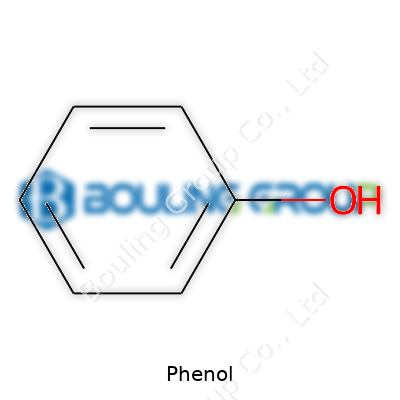
Standing alone, phenol appears as colorless or slightly pink, crystalline and with a distinct biting smell. Its formula, C6H5OH, hints at a simple structure, yet it delivers so much more. Most users first meet phenol in diluted form, whether in pharma, chemical synthesis, or classroom labs. For decades, it shaped antiseptics, bisphenol A, plastics like polycarbonates, and flavors like vanillin. No matter the field—adhesives in woodworking, herbicides on farms, prescription pain relief at the dentist—phenol’s versatility keeps it in demand.
Physical and Chemical Properties
At room temperature, phenol’s crystals look innocent. Warming up past 40°C, they melt into a transparent, mobile liquid. The odor, sweet but medicinal, cuts through the air and hints at its potency. At 182°C, those crystals boil, giving off vapors users avoid breathing. Water barely dissolves phenol, but alcohol, ether, or even warm water mix more readily with it. On paper, phenol holds one weakly acidic hydroxyl group; in practice, it corrodes tissues and stains skin. It finds itself wedged between alcohols and acids on the pKa scale. The benzene ring’s stability supports substitutions, making the molecule a regular participant in industrial chemistry. Phenol’s chemical reactions read like a shopping list for the creative chemist: halogenation, nitration, oxidation, sulfonation, and the classic Kolbe-Schmitt reaction all play out easily near this core structure.
Technical Specifications & Labeling
Manufacturers ship phenol according to strict specifications. Pure phenol surpasses 99% content, free of heavy metals and other aromatics. Impurities, especially water and tar derivatives, trigger costly headaches in downstream processes, so each shipment gets tested for iron, sulfur, and acidity. Drums and canisters bear hazard labels showing skull-and-crossbones, alerting handlers to acute toxicity and flammability. Detailed MSDS booklets follow each batch and spell out required PPE, ventilation, and first-aid measures. Tech data sheets recommend storing phenol in cool, dry, well-ventilated spaces, usually in steel containers with built-in liners to prevent leaks. Sites pouring thousands of tons into reactors every year keep digital logs and real-time monitoring so an errant leak won’t become a disaster.
Preparation and Synthetic Methods
Old factories coaxed phenol from coal tar. Modern industry leans hard on the cumene process, also called the Hock process. Here’s the gist: benzene and propylene combine to form cumene, which then oxidizes to cumene hydroperoxide. Acid cleavage breaks that molecule in two, yielding a batch of phenol and a smaller helping of acetone. This method, developed in the 1940s, beats older routes for efficiency and safety. Some sites still use toluene oxidation, but rising costs and waste volumes push those to the sidelines. A handful of smaller labs extract phenol from salicylic acid or chlorobenzene as needed for research and specialty uses.
Chemical Reactions and Modification Potential
Phenol excels at substitution. Its hydroxyl group pulls electrons into the benzene ring, so substitutions favor the ortho and para positions. Chemists use this reactivity to add nitro, sulfo, or halogen groups, or to link phenol with acids and carboxylic compounds, grafting on new chains for plastics and resins. The Kolbe-Schmitt reaction produces salicylic acid. Coupling with formaldehyde yields resins—those same resins that became the first synthetic plastics, like Bakelite. Oxidation leads to para-benzoquinone or catechol, essential compounds in dyes and photography. With each tweak, phenol’s derivatives fill new niches across chemical industries.
Aliases and Product Names
Phenol appears in documents as carbolic acid, hydroxybenzene, monohydroxybenzene, or phenyl alcohol. In trade, buyers talk about phenol flakes, liquefied phenol, or even “crystal carbolic” depending on the package. Each synonym reveals something about usage or history—carbolic hints at medicine, hydroxybenzene signals analytical chemistry, and phenol anchors the plastics sector. In international trade, its CAS number (108-95-2) flags shipments for customs checks and regulatory reviews.
Safety Measures and Handling Standards
Ask any technician about phenol, and safety stories come fast. Direct skin exposure burns within seconds. If inhaled, vapors cause coughing, dizziness, even systemic poisoning. Swallowing small amounts triggers stomach pain, vomiting, and seizures. OSHA and European directives treat phenol as a major hazard chemical, placing strict limits on airborne particles and requiring full gloves, face shields, and chemical aprons in all handling areas. Emergency showers and eyewash stations stand near every drum, not as a formality, but to save lives. Locking storerooms, limiting open transfers, and weekly safety drills all keep incidents rare. No corner of a chemical facility handles phenol casually.
Uses and Application Range
Phenol stretches its reach across dozens of industries. In resins, it binds plywood layers and glues car brakes together. Chemists shape pharmaceutical intermediates like aspirin and acetaminophen by working through phenol-based syntheses. Everyday items—from household cleaners to disinfectants—lean on diluted phenol for germ-killing action. Specialty solvents for paints and inks, weed-killing herbicides, antiseptic throat sprays, and flavor ingredients in synthetic vanillin—phenol touches them all. It even blends in as an extraction agent for laboratory DNA and RNA research. Every industry sets its own standards for purity, residue, and traceability with phenol as the backbone ingredient.
Research, Innovation, and Development Strides
Researchers use phenol as a proving ground for greener chemistry, looking to cut byproducts and improve yields. Academic labs target milder reaction conditions, enzyme-catalyzed modifications, and alternative feedstocks outside of benzene or fossil fuels. Process engineers test recycling and water treatment methods to grab lost phenol from wastewater, reducing environmental impacts and boosting profits. Polymer scientists exploit new phenolic resins for carbon-fiber composites, bio-based adhesives, and electronic coatings. Phenol remains a centerpiece of chemical innovation, with papers and patents stacking up every year.
Updates in Toxicity and Health Research
Old wisdom about phenol’s hazards guides every lab, but new studies keep pushing for more precise limits. Chronic exposure in factories links to skin disorders and organ damage, while animal studies help map safe handling doses. The drive to understand how phenol moves through water, air, and soil draws environmental agencies into long-term tracking projects. Some medical researchers connect phenol by-products with certain cancers, triggering new workplace regulations and exposure monitoring. Layering this knowledge, medical teams review antidotes and decontamination best practices, aiming to cut injuries from accidental exposure. As users and workers raise concerns, industry responds by phasing out specific formulations and launching safer alternatives.
Market Outlook and Future Prospects
Phenol’s global market keeps growing, shaped by demand for plastics, resins, and pharmaceuticals. Regulatory pressure increases the cost of waste and emissions, favoring greener production pathways and bio-based sources. More players test new routes from lignin and plant byproducts, hoping to reduce dependence on oil. Electric vehicles and future electronics boost the need for high-performance adhesives and composite materials, all building on phenol chemistry. Researchers and investors watch for breakthroughs that deliver lower carbon footprints and close chemical cycles, knowing that the old story of coal tar and early antiseptics spun out into a world of uses that keeps on expanding.
Hidden Utility in Daily Life
Most people walk through their day not giving a second thought to phenol, but this organic compound plays a bigger part in daily life than folks realize. My old chemistry professor used to joke that “if you want to understand civilization, understand phenol.” That stuck with me, probably because the more I learned, the more I saw truth in it.
Health and Hygiene Depend on It
The medicine cabinet usually gives phenol away. It pops up in throat sprays and sore throat lozenges. Years ago, I remember that weird numbing sensation after a dose of spray for a sore throat — that numbing didn’t just happen by magic. Phenol does the heavy lifting; it deadens nerves on contact and kills many bacteria. Hospitals rely on phenolic antiseptics to disinfect surfaces, so patient rooms stay safer. In the late 1800s, Joseph Lister used phenol to sterilize surgical tools, sparking huge advances in infection control. That lesson stuck, and today phenolic disinfectants still hold their own where germs run rampant.
The World of Plastics
Phenol turns up in rooms across the world — as part of the structural heart of electronics, furniture, automotive panels, and countertops. Bakelite, made from phenol and formaldehyde, launched as one of the very first plastics. It kicked off the move away from natural materials for everyday objects. Now, engineers use phenol to help make strong, lightweight composites for smartphones and durable parts in cars. These aren’t just trinkets; they help make cars safer, phones lighter, and kitchens sturdier.
Harvesting Real-World Benefits
House paints and coatings owe a lot to phenol’s chemistry. Alkylphenols, spun from phenol, increase gloss and durability in modern paints. I worked construction for a spell, and I learned to appreciate that extra year or two before repainting the trim. Foam insulation and household cleaners draw on phenol for resistance to mold and breakdown from sunlight. Even in agriculture, phenol derivatives guard plants against infections, supporting crop yields.
Safety and Solutions
Phenol, for all its usefulness, carries a toxic punch. It burns skin and damages organs at high exposure. Factory workers and lab staff can’t cut corners on safety—respirators, gloves, tight controls, and constant air monitoring remain part of the job. Some chemical factories have run into environmental trouble by dumping phenol-laden waste into rivers, leading to poisoning in fish and people. That sort of negligence sets off alarms, and for good reason. Regulations now force industry to clean and recycle waste and keep a lid on emissions.
Reducing harm means more research into greener production methods. Chemists are finding ways to use plant-based ingredients as starting points, swapping out the old petrochemical feedstocks. Some companies now recover and reuse phenolic solvents rather than burning or dumping them. Small victories, but important for neighborhoods near factories and anyone who eats fish from those rivers.
We Rely on It — Wisely
Phenol keeps showing up in places people do not expect, and life gets a bit more convenient, healthier, and safer because of it. Most folks never see those behind-the-scenes details, but it’s wise to remember the two sides of this powerful compound. It provides real value if handled with respect and responsibility.
Clear Risks Behind the Chemistry
Standing in a school laboratory for the first time, I came across a bottle labeled phenol. The label featured a few ominous skull-and-crossbones and diligent warnings. A closer look into phenol reveals these warnings serve a purpose beyond bureaucracy. Phenol isn’t some rare villain from a chemistry textbook – it finds use in everything from plastics to disinfectants, aspirin synthesis to polymer production. Its practical value cannot be denied, but ignoring its dark side leads to real trouble.
Exposure Isn’t Just Academic
Skin contact with phenol can bring severe chemical burns in minutes. Even tiny splashes, the kind easily missed in a busy lab or industrial plant, can trigger pain and skin whitening. At higher concentrations, the substance penetrates skin rapidly, entering the bloodstream. More than just a rash, this can lead to systemic toxicity that strains organs such as the heart, liver, and kidneys. Respiratory exposure, whether from spills or vapors during handling, brings dizziness, throat irritation, trouble breathing, and much worse if inhaled in large amounts.
Short-term symptoms paint one picture; the long-term effects are harder to ignore. Chronic exposure, even at low doses, sometimes links to nervous system problems and potential organ damage. The United States Occupational Safety and Health Administration (OSHA) limits workplace exposure to 5 parts per million over an eight-hour shift due to these dangers. That limit doesn't come lightly. Case histories are full of lab and factory workers suffering from careless work habits—flimsy gloves, no goggles, poorly ventilated rooms.
Knowledge Means More Than Warnings
People often trust their eyes and nose for protection: "If I can’t smell it, I’m safe." Phenol tricks both. The substance emits a sweet, medicinal smell, but olfactory fatigue sets in quickly. Workers may underestimate their exposure, especially when surrounded by familiar smells in a busy workspace.
In my experience, too many folks in both education and industrial settings gamble on thin gloves or ignore splash warnings if they're in a hurry. Not all gloves hold up. Only certain materials, like butyl rubber or neoprene, resist phenol’s corrosive nature for long without breaking down. Nitrile or latex, cheap and common, offer a false sense of security.
Practical Safety Steps Matter
The real solution isn’t a mountain of paperwork or endless safety videos. Fast access to emergency showers and eyewash stations reduces injury if exposure occurs. Workspaces using phenol should have effective fume hoods and ready antidotes like polyethylene glycol, which can limit damage by washing polluted skin. Training matters too, but it works best with demonstrations, direct conversation, and team responsibility. People remember an urgent chemistry professor dousing a burn as a live warning, much more than reading another MSDS sheet.
Tracking who handles phenol, in what quantities, and under what circumstances, helps spot trouble before hospital visits mount. Regular audits, honest incident reporting, and a culture where "speaking up" never gets ridiculed make a bigger impact than lockers full of untouched gloves.
Respect Unlocks the Value Safely
Phenol earns its place in the toolkit of industry, research, and healthcare. But respect, not fear or indifference, should guide every interaction with it. Treating phenol like a neutral bottle on the shelf invites disaster. Trading a little convenience for proper preparation saves careers and lives. This lesson isn’t limited to one chemical—it's a practical principle for anyone who steps into a lab, factory, or classroom where risks and rewards walk side by side.
Real Dangers, Real Responsibility
Walking through a lab packed with bottles and drums of chemicals, you only need one whiff of phenol to realize it’s not just another item on a shelf. Phenol is corrosive. Touching or inhaling it burns tissues and can knock out your nervous system. There’s a reason seasoned chemists stay vigilant around it. Too many accidents trace back to sloppy storage and carelessness, some with tragic outcomes. A single leak can send toxic fumes through hallways or seep into drains, putting everyone at risk. Years ago, a research friend learned this the hard way after a cracked bottle led to a shut-down and two hospital visits. Those are stories nobody wants to relive.
What Science Says About Storing Phenol
Storing phenol safely starts with picking the right container. It reacts with certain metals and plastics, so workers stick with dark, tightly-sealed glass or high-density polyethylene containers. Direct sunlight can trigger chemical breakdown and pressure build-up, so bottles deserve dark, cool spaces—with no windows in sight. Humidity only makes the risks worse, as water can promote phenol’s corrosiveness and increase accident chances.
Regulations in places like Europe, the United States, and Japan set clear rules here. The Occupational Safety and Health Administration (OSHA) highlights temperature control, ventilation, and segregation from incompatible chemicals like strong oxidizers or acids. Sweat the small stuff: one missed detail can multiply hazards fast.
The Human Element in Chemical Safety
No matter how airtight the rules, safe storage still depends on the people in the building. Training makes a huge difference. Rushing to finish a job or ignoring a spill magnifies risk. In too many labs, broken habits or shortcuts slowly creep in. Regular retraining focused on real scenarios—not just dry instructions—keeps safety routines fresh. Sharing close-call stories and walking through storage areas to spot issues before they turn into emergencies reinforces a culture of responsibility.
Clear labels and accurate inventories cut down mistakes during retrieval or disposal. Checking shelf life and rotating stock so old bottles don’t linger unnoticed in the back helps too. Removing damaged or unnecessary containers early stops incidents before they begin. Most spills or exposures result from avoidable mistakes: poor labeling, messy storage rooms, forgotten guidance notes, or plain distraction.
Solutions Grounded in Experience and Evidence
Simple fixes go a long way. Store phenol below 25°C, in a locked, ventilated cabinet marked toxic and corrosive. Place absorbent pads or chemical spill kits nearby. Maintain clear emergency showers and eyewash stations within sight of the storage room, not three corridors away. Put Material Safety Data Sheets (MSDS) next to the entry for instant reference, not buried in a computer system no one can access quickly.
Colleagues involved in chemical safety often stress routine audits. Schedule surprise inspections and yield real-world feedback, not just ticking boxes. Encourage immediate reporting of container damage or strange smells. If management listens, trust builds—and more hazards get caught early.
Remember, phenol has a place in research, production, and everyday life—from pharmaceuticals to plastics. With smart, disciplined storage and a focus on teamwork, dangerous incidents drop sharply. Trust in experience, scientific evidence, and the lessons written in regulation and in real hospital logs. That combination works every single time.
Strong Smell and Taste
The first thing people notice about phenol is the sharp, medicinal smell. It’s not the sort of thing you forget. It used to show up in old-school cough medicine and some mouthwashes, so the scent brings back memories for many of us who’ve ever stepped into a hospital supplies closet. That strong flavor and smell warn you to be careful—phenol isn’t something to splash around without protection.
Acidic Strength You Can Feel
Many folks call phenol a “weak acid,” but that doesn't really capture what’s happening in your hand or on your workbench. If it touches your skin, the burn comes soon after. It’s not as dramatic as hydrochloric acid, but the redness and pain signal there’s real chemistry happening. Drop phenol in water and it doesn’t fully dissolve, yet it does ionize a bit, making the solution acidic. In labs, scientists use this property to separate molecules or to sanitize equipment. The mix of being not too strong but strong enough gives phenol a unique place among chemicals.
Solubility Tells a Story
Water and phenol don’t mix too well. At room temperature, you can get about eight grams of phenol to dissolve in one hundred milliliters of water. Warm up the solution or add more phenol and you get two distinct layers—one clear, one bright. This property comes from the shape of the phenol molecule. A part loves water and wants to blend in, but another part—the benzene ring—shuns it. That makes chemists think twice about what they mix phenol with and changes the way factories separate and clean up phenol spills.
Reactivity and Everyday Danger
Phenol reacts fast with quite a few things. Exposed to oxygen in the air, it darkens and forms sticky stuff on the surface—good luck getting that off your workspace. Mix in some formaldehyde, and you get the base for Bakelite, one of the world’s first plastics. At the same time, phenol’s reactivity means it’s dangerous for ordinary skin. Even a drop can kill nerve endings and cause white patches. Hospitals used to use phenol in antiseptics, but better and safer options have replaced it for most jobs today.
Uses That Touch Daily Life
People don’t always hear “phenol” when they buy plastic picnic plates or laminate their countertops. Still, most household plastics, resins, and even some medicines are built on phenol. Scientists use it to break apart cell membranes for DNA extraction. Phenol is also used to make dyes, herbicides, and cosmetics. It’s a basic building block for dozens of everyday products. Knowledge about its properties isn’t just for chemists—a basic understanding helps people recognize hazards at home, school, or work.
Tackling the Hazards
Working safely with phenol starts with respect for its properties. Gloves, glasses, and good ventilation don’t just keep inspectors happy— they keep peace of mind. From childhood chemistry sets to big manufacturers, I’ve seen what happens when someone underestimates phenol. Burns heal, but awareness sticks even longer. Good storage, careful waste disposal, and constant training are critical steps to prevent accidents and protect the environment. Public health and safety matter in every setting—clear labeling and education belong at the top of the checklist.
Understanding Phenol’s Role in Disinfection
Phenol’s story goes way back in the history of medicine and sanitation. Joseph Lister used phenol, which folks often called carbolic acid, during surgeries in the 1800s to keep wounds clean and infections at bay. Medical textbooks point to phenol as the original chemical disinfectant. It earned its reputation by breaking down cell walls and stopping bacterial growth almost on contact. Until antibiotics came along, hospitals leaned heavily on this strong-smelling chemical.
Why Phenol Works—And What to Watch For
Phenol can kill bacteria, fungus, and even some viruses. It does this by damaging the proteins inside those unwanted germs. Lab tests show that phenol wipes out Staphylococcus and Streptococcus, which often cause nasty infections. Some public health facilities still turn to phenol, especially for areas with a lot of organic mess or when dealing with hardy bacteria strains.
But phenol demands respect. The liquid burns skin and can cause headaches, dizziness, and kidney trouble if someone breathes in the fumes for too long. A drop on the skin quickly numbs and turns white, a warning sign from your own body. Swallowing even a small dose spells disaster for organs. Not all cleaning jobs call for something so powerful or risky, and everyday use around homes or schools isn’t the best plan.
Modern Choices and Health Impacts
Many commercial disinfectants today have gentler formulas. Hydrogen peroxide and alcohol get the job done for household surfaces without the same hazards. Hospitals used to rely on “phenolic” cleaners, but better alternatives replaced them. The Centers for Disease Control and World Health Organization both recommend easier substances because they put fewer people in harm’s way. Common sense tells you not to reach for a sledgehammer when a screwdriver will do, especially if kids or pets live where you clean.
Nobody wants to trade bacteria for dangerous fumes. Occupational safety regulators list phenol as a chemical worth watching. In places that still need phenol—for example, high-security biomedical labs—workers wear gloves, goggles, and sometimes respirators. Most hardware stores won’t even sell full-strength phenol to regular shoppers, for good reason.
What Makes a Good Disinfectant?
People care about thorough cleaning, but also about personal safety. Market shelves fill up with new disinfectants advertised as “green” and “child-safe.” It’s tempting to chase those labels, but checking up on ingredients and credentials still matters. The Environmental Protection Agency keeps a registry of antimicrobial products that really stand up to germs.
Sterile doesn’t mean risky. Soap, alcohol, and diluted bleach do well for most chores. Vanquishing germs takes more than brute force—it means considering side effects, understanding the risk to family and pets, and picking products backed by evidence. Phenol won its place in history, but homes and hospitals benefit from fresh solutions.
Safer Paths Forward
If your goal is truly clean surfaces, smart habits outweigh any one chemical. Washing hands beats chasing germs with a toxic spray. Touching up high-traffic spots with proven, safer products gives peace of mind without risking a trip to the ER. The best disinfectants get trusted by experts, not just because they work, but because people can use them without fear.
| Names | |
| Preferred IUPAC name | benzenol |
| Other names |
Carbolic acid
Hydroxybenzene Phenic acid Phenylic acid Monohydroxybenzene Benzenol Oxybenzene |
| Pronunciation | /ˈfiː.nɒl/ |
| Identifiers | |
| CAS Number | 108-95-2 |
| Beilstein Reference | fourth edition, III, 199 |
| ChEBI | CHEBI:15882 |
| ChEMBL | CHEMBL143 |
| ChemSpider | 969 |
| DrugBank | DB03255 |
| ECHA InfoCard | 100.003.561 |
| EC Number | 1.14.13.7 |
| Gmelin Reference | 1995 |
| KEGG | C00123 |
| MeSH | D010668 |
| PubChem CID | 996 |
| RTECS number | SJ3325000 |
| UNII | 4T9Y884Y6X |
| UN number | 1671 |
| Properties | |
| Chemical formula | C6H5OH |
| Molar mass | 94.11 g/mol |
| Appearance | Colorless or white crystalline solid with a distinct odor |
| Odor | Distinct, sickly sweet odor |
| Density | 1.07 g/cm³ |
| Solubility in water | 84.2 g/L (20 °C) |
| log P | 1.46 |
| Vapor pressure | 0.4 mmHg (20°C) |
| Acidity (pKa) | 9.95 |
| Basicity (pKb) | 9.89 |
| Magnetic susceptibility (χ) | -47.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.542 |
| Viscosity | 10 – 20 cP |
| Dipole moment | 1.70 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 144.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -165.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3054.0 kJ/mol |
| Pharmacology | |
| ATC code | D08AD03 |
| Hazards | |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled; causes severe skin burns and eye damage; may cause respiratory irritation. |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS02, GHS06, GHS08 |
| Signal word | Danger |
| Hazard statements | H290, H301, H311, H314, H331, H341, H373 |
| Precautionary statements | P260, P262, P264, P270, P271, P280, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P308+P311, P310, P321, P330, P363, P405, P501 |
| NFPA 704 (fire diamond) | 3-2-2-Acid |
| Flash point | 79 °C |
| Autoignition temperature | 715°F (379°C) |
| Explosive limits | 1.3–9.5% |
| Lethal dose or concentration | LD50 oral rat 317 mg/kg |
| LD50 (median dose) | LD50 (median dose): 317 mg/kg (oral, rat) |
| NIOSH | UN1671 |
| PEL (Permissible) | 5 ppm |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | 250 ppm |

