Propanoic Acid: A Practical Commentary on its Development, Use, and Future
Historical Development
Propanoic acid, once called propionic acid, first drew the attention of chemists in the 19th century. A German chemist, Johann Gottlieb, discovered it in 1844 through the distillation of wood. Early scientists kept improving their techniques, eventually producing it in greater quantities by fermenting sugars and using petrochemical processes. As synthetic organic chemistry matured, producers leaned into petrochemical methods, especially since propanoic acid turned out valuable far beyond its odd, strong smell. The move to industrial-scale production opened up several uses and gave researchers the material they needed for deep dives on its properties and safety.
Product Overview
Propanoic acid now lands mainly in the food, animal feed, and chemical industries. Its pungency sometimes overshadows its bigger agricultural and chemical punch. Manufacturers look for its dependable role as a preservative, keeping bread and animal feed from spoiling and keeping mold at bay. The chemical sector often uses propanoic acid to make herbicides, pharmaceuticals, and cellulose-based plastics. Across packaging and product safety, the material gets labeled with its correct name, clear concentration information, and hazard details per global standards, especially since handling it calls for serious care.
Physical & Chemical Properties
Propanoic acid takes the form of a colorless, oily liquid with a pretty sharp, unpleasant odor. The acid boils at around 141°C and freezes at just under 0°C. Its solubility in water and organic solvents helps in countless application setups. Chemically, it carries a moderate acidity (pKa near 4.87), letting it act both as a precursor in chemical reactions and as a mildly corrosive preservative. It carries three carbon atoms, balancing accessibility with just enough reactive punch for synthetic work, especially where a small, carboxylic acid fits the job.
Technical Specifications & Labeling
Bulk suppliers list it under CAS No. 79-09-4, highlight purity (typically above 99% for food and lab standards), and spell out regulatory categories for shipping and storage. Packaging comes with corrosion warnings, flammability notices, and storage advice to keep the acid cool and containers tight. Labels also identify whether contaminants like water, acetic acid, or higher-boiling impurities slip in, since food and pharma products carry extra regulatory burdens. The specifications ensure buyers can match the acid to their needs, while plant workers, drivers, and lab folks know what they’re handling.
Preparation Method
Industrial methods lean on hydrocarboxylation, where ethylene gas combines with carbon monoxide and water using a cobalt or rhodium catalyst, or on direct oxidation of propionaldehyde (itself made by hydroformylation of ethylene). Researchers have revived fermentation with propionibacteria to tailor “natural” acidity for some food applications, since certain markets want a non-petrochemical route. Small labs sometimes use oxidation of primary alcohols (like 1-propanol) to create modest amounts for study or specialty work. Each process creates a strong-smelling liquid that distills over and needs careful separation from water and byproducts.
Chemical Reactions & Modifications
Chemists like how propanoic acid can transform. Its carboxyl group makes esterification straightforward: reacting with alcohols produces esters that show up in fragrances and solvents. Conversion to its acid chloride, through thionyl chloride or phosphorus trichloride, opens access to further derivatives—like amides or anhydrides—used in pharmacy and peptide synthesis. As a three-carbon building block, propanoic acid lets experts stitch up more complex molecules or serve as a model substrate for enzyme and catalysis studies. The direct reactions with bases, reduction to propanol, and halogenation mark other favorite laboratory applications.
Synonyms & Product Names
You’ll see “propionic acid,” “ethylformic acid,” or “carboxyethane” on older papers and some global labels, but most industries use “propanoic acid.” Systematic nomenclature gives way to trade names depending on producer and market—many food-grade variants carry customized names, while feed and industrial chemicals stick to recognizable chemical identifiers for ease of international commerce. Every safety sheet uses the IUPAC name and the CAS number for global consistency, and customs paperwork follows suit to avoid mishaps.
Safety & Operational Standards
Propanoic acid calls for respect in every setting. Its vapor can irritate eyes, nose, and lungs, and liquid burns skin. Industry practices involve solid ventilation, acid-resistant gloves, splash goggles, and, in some cases, vapor masks. Storage containers need to resist corrosion, and accidental release plans line up with localized fire response, since the acid can catch fire above its flashpoint (around 54°C). International bodies like OSHA, the ECHA, and China’s GB standards spell out permissible exposure limits and medical response guidance; every plant worker gets trained in those routines. Anyone using it near food, feed, or pharmaceuticals works under even stricter regimes, with batch records and traceability, since accidental overdosing brings health risks.
Application Area
Bread makers rely on propanoic acid to stretch shelf life and push back mold, letting companies send their products farther without spoilage. Livestock feed operations use it to cut losses from mold and bacteria that could otherwise lead to sick herds or wasted crops. On the chemical synthesis side, companies fine-tune herbicides, pharmaceuticals, and solvents, counting on the acid’s three-carbon backbone. Cellulose plastics, once common in film and packaging, can also trace part of their chemistry back to propanoic acid as a building block. Some researchers turn to propanoic acid as they search for greener synthesis methods, looking for alternatives to older, more toxic chemicals.
Research & Development
Academic and industry teams still comb over propanoic acid’s chemical behavior. I’ve noticed a push around bio-based routes, since bio-acids often fetch a premium from buyers looking for eco-labels or plant-based claims. Research also digs into more selective catalysts, aiming for less waste and higher yields in each step. Drug designers use propanoic acid as a testbed for targeting certain enzyme families, since its simple structure lets them spot mechanism details before moving to bulkier acids. In materials science, the acid sometimes pops up as a modifier for specialty polymers or surface coatings, showing how an old chemical keeps finding new angles.
Toxicity Research
Recent toxicology reviews show propanoic acid’s low acute toxicity by ingestion or skin contact, but prolonged or repeated exposure can damage skin, eyes, and mucous membranes. Some studies investigate metabolic impacts from chronic low-level exposure in animal feed, since changes in gut microflora could affect nutrient uptake or animal health. Long-term atmospheric monitoring focuses on air quality in food plants using open vats, keeping worker health in focus. Regulatory agencies keep updating safe exposure levels, especially as new animal studies sharpen the picture. Misuse, accidental spills, or mistakes in handling can still lead to severe burns or respiratory distress, which is why every lab and food plant keeps antidote eye stations and emergency wash facilities on hand.
Future Prospects
Chemical makers have turned a sharp eye toward cleaner propanoic acid routes, aiming at bioprocessing with engineered bacteria or greener catalysts. More feed and food companies expect “bio-acid” labeling to catch fire, pushing for full traceability from cornfield or sugarcane to finished loaf. Safety and monitoring tech keep improving, letting producers deposit less vapor into the air and cut contact risk for workers. Synthetic chemists still chase higher margins via new esters or derivatives, using propanoic acid as both raw material and a test case for greener chemistry applications. As society keeps looking for both better preservation and lower environmental impact, propanoic acid stays important in this evolving balance.
What Propanoic Acid Means to Everyday Life
Farmers and bakers might not hang out together, but both groups benefit from the same clear liquid—propanoic acid. This compound pops up in food preservation and agriculture, making it a low-profile hero for public health and food safety. If you’ve ever tossed out a moldy loaf of bread, you’ve witnessed a fight propanoic acid helps avoid.
Fighting Spoilage in the Food Chain
For bread and other baked goods, mold is a big headache. Propanoic acid tackles this problem head-on. It stops bacteria and mold from taking over, letting bakery shelves stay stocked a little longer and reducing food waste. Extending the lifespan of food might seem like a technical victory, but for families on tight budgets or regions where food deliveries come few and far between, it has daily significance.
Companies use propanoic acid for its effectiveness in stalling spoilage without covering up flavors. You can find it on ingredient labels as calcium propionate or sodium propionate—forms that work just as hard. The U.S. Food and Drug Administration and European Food Safety Authority review additives like these, keeping consumers in the loop about benefits and safe exposure levels.
Helping Farms Feed the World
Picture a silo packed with damp grain. Add in high temperatures, and you get a playground for molds. These molds create toxins that hit livestock health and, down the line, the quality of meat, milk, and eggs. Propanoic acid comes in with a straight-up solution: bring moisture and bacteria under control and cut down the risks. As a result, more grain ends up as feed, not trash.
Farmers use it to keep stored hay and silage fresh. It keeps livestock feed from spoiling before animals even get to eat it. This lift in feed quality not only improves animal health but also strengthens food security, which has ripple effects reaching well beyond the farm.
Industrial Roles You Might Not Expect
People rarely mention propanoic acid outside of agriculture and food talks, but its chemical skills stretch even further. It plays a supporting role in making herbicides, pharmaceuticals, and some plastics. Everyday items, from weed control sprays in gardens to the coatings on a pill, owe a small thanks to this acid.
Each step in these processes relies on regulations and careful handling. Factories train workers to avoid skin contact and inhaling fumes because, like many chemicals, unchecked exposure can cause trouble. Responsibility at this stage means both stronger safety for workers and less risk of environmental fallout.
Keeping Health and Safety Front and Center
Nothing in chemistry comes risk-free. Good science means acknowledging both the upsides and hazards. While propanoic acid has a solid record as a preservative, some groups keep an eye on studies about potential allergies or sensitivities. Health professionals want long-term safety for everyone. Public access to product ingredient lists and ongoing research help people avoid known issues and push for better protections.
Building a Smart Path Forward
Food waste stacks up at billions of pounds per year. Chemical preservatives aren’t the only answer, but ignoring their impact doesn’t help. Combining clean technology with food safety keeps both shelves stocked and consumers safe. More research, open discussions between scientists, regulators, and the public, and better education for food workers and farmers can help guide smarter choices. Safe storage and handling keep workplaces and products on the right track—and ensure that compounds like propanoic acid stick around as helpers, not hazards.
A Look at Propanoic Acid in Everyday Settings
Propanoic acid turns up in more places than most folks expect. You’ll spot it on the ingredient list of bread as a preservative. Manufacturers add it to prevent mold and bacteria from making food go bad. In industry, the stuff finds its way into plastics, herbicides, and even perfumes. Many people who’ve worked in food processing, chemistry labs, or agriculture have crossed paths with propanoic acid. I first ran across it back in a high school lab, measuring out its slightly sharp liquid under a fume hood. The smell—resembling sour milk—left a strong memory.
Safety in Handling: The Human Side
Working with chemicals doesn't need to be frightening, but it pays to know the risks. Propanoic acid, despite its food-world appearance, brings very real hazards. It can cause burns if it touches the skin or eyes. Breathing in its fumes irritates airways and lungs. People who've splashed it on themselves may remember the sting and redness that follows, especially on sensitive skin. Accidents usually come down to skipping gloves or goggles, hurrying, or believing “it’s just food grade, so it can’t hurt me.”
At concentrations found in commercial barrels or lab bottles, contact with propanoic acid should always be taken seriously. The American Conference of Governmental Industrial Hygienists (ACGIH) sets a threshold limit value of 10 ppm for workplace air—enough to keep the air clear and workers safe. In my own teaching, basic safety gear like splash goggles, chemical-resistant gloves, and a lab coat formed the starting point. Chemical-resistant aprons help keep larger spills off clothing and skin. Above all, good air movement matters. A strong exhaust fan or a fume hood keeps vapors away from faces and lungs.
Lessons Learned and Solutions for Safer Work
Too often, accidents show up after years of letting small risks slide. Stories get told about “the time I didn’t bother with gloves and regretted it.” Proper training and a culture that respects the hazards make all the difference. Supervisors must regularly review safety rules and demonstrate gear use rather than assuming everyone knows the drill.
Storage also plays a major role. Propanoic acid must stay tightly sealed in containers—nobody enjoys cleaning up a spilled acid that eats through shelving. Labels on bottles help newcomers recognize what’s inside, avoiding mix-ups during busy shifts. In places where acid exposure might happen, having a clear plan for rinsing skin or eyes can make a world of difference. An eyewash station or emergency shower stands ready to minimize long-term damage, provided people know where it is and how to use it.
Looking Forward: Continuing the Conversation on Safety
If your work or studies take you near propanoic acid, view it with respect. No matter the purity or packaging, the same precautions apply. Watch out for short cuts. Make safety gear a habit, teach routines by example, and keep safety info visible. Propanoic acid can be handled safely if everyone on the team shares responsibility—and no one assumes it’s “just another food additive.”
Understanding Propanoic Acid’s Nature
Propanoic acid comes with a sharp smell and a history of use as a preservative, but the way it behaves gives clues about how to store it. This substance has a habit of releasing fumes that irritate skin, eyes, and lungs. It also reacts with strong oxidizers and some metals, so sloppy storage can lead to more than just a harsh whiff in the air.
Why Storage Conditions Matter
I once unloaded a shipment in a warehouse where an open drum of propanoic acid sat by a loading door. The fumes made my eyes water, and staff wore respirators even for a quick peek. Such moments show that air-tight, chemical-resistant containers matter, not just for shelf-life but for safety from exposure. Quality control often means nothing if a liquid eats through a lid or sends vapors into shared airspace; it’s easy to forget about fumes until they sting your nose or throat.
Stable Temperatures, Real People
This acid gives off vapors even at room temperature, so store it away from direct heat, sunlight, or fluctuating climates. Stainless steel or high-density polyethylene holds up well; cheap plastics or corroding metal spell trouble over months or years. A temperature-controlled storage area—a simple cool, dry spot—reduces pressure from building inside drums or containers. Sudden heat waves in an unventilated space can lead to swelling or leaks, something I’ve seen cause frantic calls for containment crews.
Clear Segregation Works Best
Some warehouse teams assume chemicals can share a shelf, but propanoic acid doesn’t like company. It reacts with oxidizing agents and certain metals—think sodium hypochlorite or rusted steel. Chemicals locked behind separate cabinets or in assigned chemical rooms make for a safe routine. Safety advocates push for marked areas and solid rules against storing acids and bases side by side. Taking shortcuts costs more than dollars if vapors mix or corrosion joins the party.
Labeling With Care
Mislabeling leads to costly mistakes, especially for smaller labs or businesses lacking chemical specialists. Lab-grade labels share hazard symbols and concentrations, not just generic names. I’ve thrown out barrels for lacking clear labels, knowing confusion can hurt new hires or visiting inspectors. That extra round of labeling and logging every new shipment pays off in peace of mind—nobody wants “mystery substance” as a line item in inventory.
Ventilation Isn’t Just a Buzzword
Good ventilation solves more than stuffiness. In spaces where propanoic acid sees regular use, ventilation hoods or simple exhaust fans push fumes outside and keep indoor air safer. It’s easy for a building manager to overlook air exchanges, but staff health can decline fast with repeated exposure. Investing in proper airflow keeps headaches and sore throats off the daily schedule. Room sensors and regular checks help catch leaks long before they turn into emergencies.
Spill Kits Aren’t Optional
Small leaks, drips, or a toppled drum need a fast response. Spill kits—including gloves, goggles, and absorbent materials—should sit within easy reach, not stashed in a distant supply closet. Quick reactions contain hazards before they reach walkways or drains. Even careful crews sometimes deal with containers that break a seal during a bumpy delivery truck ride. Practicing regular spill drills takes the mystery out of emergency response and builds confidence across the team.
Summing Up with Simple Steps
Safe storage for propanoic acid comes down to good containers, safe surroundings, smart labeling, and real preparedness. These priorities reflect respect for both the chemical’s properties and the people handling it. Hands-on experience reveals gaps in protocol faster than any policy handbook. By staying alert and organized, anyone handling propanoic acid lowers risk and keeps operations running smooth and safe.
Propanoic Acid: A Simple Molecule with Big Relevance
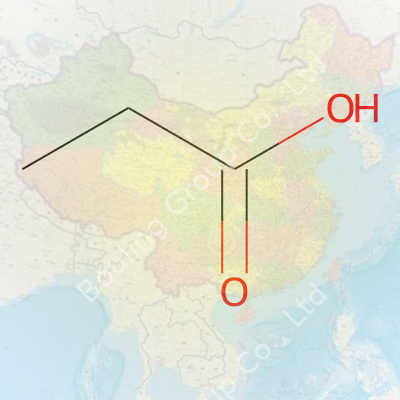
Propanoic acid fits right into that fascinating group of chemicals known as carboxylic acids. Its chemical formula, C3H6O2, might look simple at a glance, but it tells a story that matters for health, industry, and even the food on our plates. Getting to know molecules like this doesn’t call for a chemistry textbook. With some real-life context, anyone can see why these building blocks stay so important.
Where Science Meets Daily Life
Propanoic acid appears more often in the world than most people might guess. This colorless, sharp-smelling liquid pops up every time a loaf of bread resists mold or a cheese sits on the shelf without spoiling too soon. The food industry relies on its anti-mold power. That’s why you often see it or its salts—calcium propionate, for instance—on ingredient labels. Research from the Food and Agriculture Organization confirms its effectiveness in prolonging baked goods’ shelf life and adds that it keeps food waste down.
Human Health and Natural Origins
This acid isn’t just in food factories. Our own digestive system produces it every single day. When gut bacteria break down certain fibers, propanoic acid forms as a byproduct. Scientific work led by British and American microbiologists in the past decade links it to better gut health and shows it might even help reduce inflammation in the body. Overlooked nutrients like fiber owe part of their reputation to acids like this, which indirectly support our immune system.
Uses Beyond the Grocery Store
Apart from bread and cheese, propanoic acid tackles all sorts of practical jobs. Farmers use it to keep animal feeds free from fungal growths that can ruin whole silos of grain. In the manufacturing world, this same acid helps turn out herbicides and some plastics. That versatility comes from the same straightforward structure: one carboxyl group (COOH) hooked to a two-carbon tail. Chemists use propanoic acid as a starting material for more complex molecules, banking on its reactive nature to build what industry demands.
Challenges and Smarter Solutions
Of course, widespread use brings up environmental questions. Some studies point to possible negative effects if large amounts seep into waterways or soil, especially since it can lower pH and change how microbes and plants grow. Keeping chemical residues out of food and farm waste streams should matter as much as profit or shelf life. Smart policy means limiting unnecessary use and keeping an eye on how waste gets managed. Food producers in Europe already face tighter rules on just how much preservative goes into bakery products. These types of steps help balance modern convenience with environmental responsibility.
Why Propanoic Acid Matters
Learning the formula C3H6O2 is only the start. The more you look, the more these small pieces fit into the bigger picture—how science shapes what we eat, how we grow food, and how we protect environments for everyone. Getting curious about a single molecule opens doors to new ways of solving the kinds of problems that touch us all.
Recognizing the Risks in the Workplace
Propanoic acid turns up in chemical plants, labs, and even food manufacturing sites. It serves as a preservative and a building block for other compounds. Most people won’t run into it in daily life, but anyone responsible for handling or storing propanoic acid can’t afford to treat spills lightly. Propanoic acid gives off sharp fumes that sting the eyes and nose. Direct skin contact causes burns, and breathing too much vapor makes it hard to breathe. Real risk shows up in places where workers forget their gloves, or where old pipes drip into corners nobody checks.
Reacting Fast without Panic
Picture the scene: a drum tips over, escaping liquid pools near a busy area. Doing nothing invites fumes that can harm people and corrode nearby metals. Every second matters. First responders need to clear unaffected workers away right away. At the same time, they pull on proper gloves, splash-proof goggles, and chemical suits. For smaller spills, absorbent pads suck up the liquid before it can flow into drains. Larger mishaps call for neutralizers, such as sodium carbonate, poured right onto the acid. Plenty of guidelines—even OSHA’s—spell out these steps, but the real skill comes from crews who drill for spills and know their equipment like the back of their hand.
Tools and Substances that Actually Work
Cleanup teams shouldn’t reach for paper towels or mops. Propanoic acid tears through thin materials and sends vapors floating up fast, especially in cramped rooms. Industrial absorbents, such as spill control granules, lock up the acid before it spreads any farther. Ventilating the cleanup spot reduces toxic air. Open windows, turn on exhaust fans—simple habits make a huge difference. After neutralization, dispose of all wet material in sealed containers designed for hazardous waste. Disposal teams at licensed centers know how to treat these remains, which rules out dumping them with everyday trash.
Practical Habits that Prevent Trouble
In jobs I’ve held at chemical warehouses, nothing beats keeping storage drums tight, clean, and labeled. Managers need to keep inventory low. Train every hire—not just the crew on chemical duty. Practice drills for spills so everyone’s reflexes kick in, not just their memories. Check emergency showers and eyewash stations every week instead of taking their presence for granted. If a site has drains or open soil nearby, block them with spill barriers ahead of time. Regular refresher courses let new and old staff face emergencies with steady hands.
Better Communication and Record-Keeping
After any incident, responsible managers log every detail: time, location, how much escaped, how it was cleaned, how people responded. These records spark improvements, not just in manuals, but in the habits of every shift. Reports let experts pinpoint weaknesses—missing gloves, slow alarms, confused staff. Passing on lessons from mistakes keeps future teams safer. As an added layer, site managers talk with local firefighters to review plans for worst-case scenarios. Preparation always works better than regret.
Reducing Harm to People and the Planet
If a spill runs outside, both water and soil pay the price. Even small amounts hurt plants, fish, and microbes. For this reason, every site using propanoic acid should invest in both prevention and ready-to-go plans. Technology helps—real-time air monitoring or automated shutoff valves often stop problems long before the human eye can see them. Honest conversations with workers and neighbors keep everyone alert and prepared, not just the factory floor.
Learning from Real Incidents
Stories stick longer than statistics. In one plant, my team contained a leak just in time, because quick training meant everyone knew their station and moved without missing a beat. No substitute for lived experience, but every company can create a safety culture where every employee recognizes risks, takes them seriously, and reacts faster than fear can set in.
| Names | |
| Preferred IUPAC name | propanoic acid |
| Other names |
Propionic acid
Ethylformic acid Methylacetic acid Propanoate acid |
| Pronunciation | /ˌprəʊ.pəˈnoʊ.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 79-09-4 |
| 3D model (JSmol) | `/showmol.cgi?mol=propanoic%20acid` |
| Beilstein Reference | Beilstein 1718732 |
| ChEBI | CHEBI:30779 |
| ChEMBL | CHEBI:30769 |
| ChemSpider | 727 |
| DrugBank | DB00163 |
| ECHA InfoCard | ECHA InfoCard: 024-002-00-6 |
| EC Number | EC 200-834-7 |
| Gmelin Reference | Gmelin Reference: **787** |
| KEGG | C00163 |
| MeSH | D017209 |
| PubChem CID | 1032 |
| RTECS number | UE5950000 |
| UNII | 9QVP80P3A5 |
| UN number | UN1844 |
| CompTox Dashboard (EPA) | propanoic acid (DTXSID9020082) |
| Properties | |
| Chemical formula | C3H6O2 |
| Molar mass | 74.08 g/mol |
| Appearance | Colorless liquid with a pungent odor. |
| Odor | Pungent, unpleasant |
| Density | 0.993 g/cm³ |
| Solubility in water | Miscible |
| log P | 0.33 |
| Vapor pressure | 3.7 mmHg (20 °C) |
| Acidity (pKa) | 4.87 |
| Basicity (pKb) | pKb ≈ 11.1 |
| Magnetic susceptibility (χ) | -49.0e-6 cm³/mol |
| Refractive index (nD) | 1.387 |
| Viscosity | 1.21 mPa·s (25 °C) |
| Dipole moment | 1.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 103.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −487.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1527.0 kJ/mol |
| Pharmacology | |
| ATC code | A03AB05 |
| Hazards | |
| GHS labelling | **GHS labelling of Propanoic Acid:** "GHS02, GHS05, GHS07, Danger, H226, H314, H335 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H314, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P264, P280, P303+P361+P353, P305+P351+P338, P370+P378, P403+P235 |
| NFPA 704 (fire diamond) | 2-3-2-Acidos |
| Flash point | 54 °C |
| Autoignition temperature | 485 °C |
| Explosive limits | 2.1–12.1% |
| Lethal dose or concentration | LD50 (oral, rat): 3450 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 3,450 mg/kg |
| NIOSH | WI8090000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Propanoic Acid: "10 mg/m³ |
| REL (Recommended) | 200 ppm |
| IDLH (Immediate danger) | 150 ppm |
| Related compounds | |
| Related compounds |
Acetic acid
Butyric acid Formic acid Valeric acid |

