Triethanolamine: A Closer Look at a Chemical Mainstay
Historical Development
Triethanolamine saw its roots during the early 20th century when chemists started tinkering with blending the properties of alcohols and amines. At first, the focus was on capturing the value of this molecule in making soaps and textiles. Industrial chemists caught onto its versatility and never let go. Over decades, it found homes in personal care, metalworking, and a range of detergents. Its progression mirrors the story of chemical innovation, where hard-to-pronounce molecules keep cropping up in everyday life—from shaving creams in the post-war years to modern-day concrete additives. By the late 1950s, large-scale production allowed it to flood multiple markets, strengthening its reach far beyond original expectations.
Product Overview
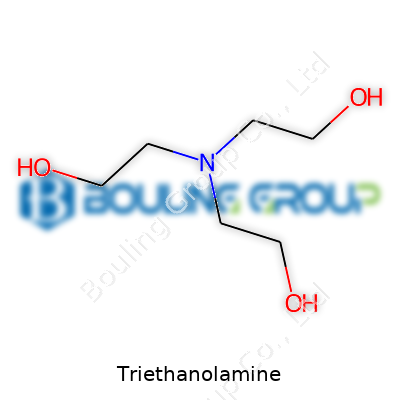
Triethanolamine, often abbreviated as TEA, carries the formula C6H15NO3. It wears many hats, acting as a pH adjuster, emulsifier, and corrosion inhibitor among others. In stores and plants alike, it often appears as a clear, viscous liquid that holds a faint ammonia odor. Producers refine it for batch consistency and purity, chasing better standards as demand grows. Between household use and industrial application, TEA’s identity constantly shifts, but its core chemical attributes keep things steady.
Physical & Chemical Properties
TEA holds a boiling point hovering near 335°C and a melting point tucked above 20°C. As a hygroscopic compound, it eagerly attracts water from its environment. The molecule’s three hydroxyl groups give it flexibility for chemical reactions and an affinity for stabilizing emulsions. Its moderate solubility profile makes it a solid choice where both oil and water phases need wrangling. The viscosity remains substantial at room temperature, and it resists decomposition under ordinary storage conditions. This stability, mixed with its slightly alkaline nature, gives manufacturers more freedom in processing and formulation.
Technical Specifications & Labeling
On commercial drums and bottles, labels flag triethanolamine concentrations (commonly above 98% for industrial use), water content, and relevant purity indices. Reputable suppliers keep analysis certificates available for every batch, confirming limits for impurities such as diethanolamine and monoethanolamine. Labeling also displays transportation and safety hazard codes, as international guidelines require. Storage instructions highlight the product’s tendency to absorb moisture, reminding handlers to seal containers tightly. In compliance with GHS and REACH, every shipment comes with detailed material safety data sheets outlining potential hazards, first aid measures, and environmental persistence.
Preparation Method
Most industrial TEA comes from the reaction between ethylene oxide and aqueous ammonia. Adjusting the ratio of these ingredients can shift the output among mono-, di-, and tri-ethanolamines. In an exothermic reaction, ammonia acts on ethylene oxide to produce a mix of products, which then pass through a series of distillation columns to reach the desired purity and specification. Plants install corrosion-resistant vessels due to the caustic nature of some intermediate steps. Streamlining temperature and pressure plays a huge role in maximizing yield and minimizing byproducts.
Chemical Reactions & Modifications
Triethanolamine reacts readily with acids to produce salts, making it effective as a buffering agent in complex solutions. It forms fatty acid soaps by esterification, laying the groundwork for its place in creams and lotions. TEA participates in alkylation, sulfonation, and other modification reactions, which chemists exploit to design surfactants, corrosion inhibitors, and textile softeners. Its tertiary amine group supports further chain extension and functionalization, which opens possibilities in polymer production. With its reactive centers, TEA provides a handy platform for synthesizing varied chemical solutions that ease operations across different sectors.
Synonyms & Product Names
Industry professionals and researchers may call it TEA, Trolamine, 2,2',2''-Nitrilotriethanol, or adjust the name to suit regulatory jargon. Trade names vary by supplier, but most packaging flags either the chemical formula or a recognizable common name. In technical literature, triethanolamine appears as both a pure chemical and under blended product titles. Suppliers maintain data sheets cross-referencing these names to ensure transparent communication.
Safety & Operational Standards
Consistent with current workplace regulations, handling TEA means wearing goggles and gloves and working in vented areas. Direct contact brings risks of mild skin and eye irritation, and inhaling vapors for long periods poses respiratory concerns. In the plant, spill control protocols and chemical-resistant containers limit the chance of leaks and splashes. Storage calls for mild temperatures away from oxidizing agents. Teams regularly review guidance from OSHA, NIOSH, and the European Chemicals Agency, while updated toxicology studies steer safe exposure levels. Workers rely on detailed training and clear signage to steer clear of risky practices and accidental misuse.
Application Area
You spot TEA in shampoos, shaving creams, metalworking fluids, concrete admixtures, and textile lubricants. In the cosmetic world, it buffers pH to gentle levels, ensuring the skin-friendly feel of lotions and gels. Metal processing plants appreciate its ability to block corrosion thanks to its film-forming powers. The concrete industry has adopted TEA to adjust setting time, making construction more adaptable under harsh weather. Even in photography and laboratory settings, TEA acts as a stabilizing and dispersing agent. Many of today’s water-based paints depend on it for improved pigment blending and smoother application.
Research & Development
Chemists and engineers keep tweaking TEA to carve out new uses and reduce possible risks. In labs, modified triethanolamine derivatives turn up as catalysts in organic synthesis and as part of novel drug-delivery systems. The push for greener chemistry has researchers exploring bio-based raw materials for TEA production, aiming to cut down fossil fuel reliance. R&D teams track regulatory changes around nitrosamine impurities, searching for tighter controls and alternative manufacturing routes. New blends and co-formulations strive to boost performance without trading away safety or environmental responsibility.
Toxicity Research
Long-term health studies on TEA have focused on dermal and inhalation toxicity. Animal tests support low acute toxicity, but repeated or excessive contact can produce mild skin sensitization. Certain impurities, especially nitrosamines formed under acidic conditions, have sparked regulatory focus due to their potential carcinogenicity. Researchers in toxicology keep an eye on chronic exposure risks in both manufacturing and end-user environments. Regulatory bodies worldwide adjust their guidance as new data emerges, balancing practical use with limits on concentration in consumer products and workplace controls. The cosmetic industry, in particular, enforces tight impurity thresholds to prevent harmful exposure.
Future Prospects
The outlook for triethanolamine hinges on trends in sustainability, regulatory compliance, and technical innovation. Cleaner manufacturing processes could shift the market toward greener TEA made from renewable feedstocks. Stricter regulations in cosmetics and construction may narrow acceptable uses, but open doors to safer, high-purity versions. As demand grows across emerging economies, producers need to balance scale-up with tighter environmental and health controls. The chemical’s core flexibility keeps it in play across familiar and emerging applications—think biodegradable detergents, novel emulsion systems, and eco-friendly inhibitors for infrastructure. Scientists continue mapping out safer synthesis routes and new functional derivatives to push TEA beyond today’s boundaries.
Everyday Products and How They Rely on Triethanolamine
Walk down any drugstore aisle and pick up a bottle of shampoo, shaving cream, or moisturizer. Flip it over and read the ingredients. Chances are, you’ll find triethanolamine listed there, tucked among a bunch of long chemical names. Over the years, I’ve learned not to overlook the impact of ingredients like this, especially ones that seem to pop up everywhere once you notice them. Triethanolamine gets its time in the spotlight for good reason. It shows up in dozens of household things for purposes that go beyond “just an additive.”
Triethanolamine usually steps in to help balance the pH of products. Skin doesn’t cooperate well when soaps or lotions are too acidic or too basic. Manufacturers use this compound to nudge the pH into that safe, skin-friendly middle ground. If you’ve ever dealt with irritated skin after using a cheap face wash, you’ve seen what happens when a formula skips this step. Proper pH maintenance can keep complicated skin flare-ups at bay. According to the U.S. Food and Drug Administration, controlling irritants and stability in cosmetics remains crucial for consumer safety. Triethanolamine delivers reliable results in that respect.
Cleaning Power That Doesn’t Wreck the Surface
Beyond grooming goods, triethanolamine proves its worth in household and industrial cleaning products. Whether you’re scrubbing countertops or working with floor cleaners in a commercial space, you want a solution that removes grime without damaging the surface underneath. This compound helps detergents break down oils and dirt. It also keeps active ingredients evenly dissolved and ready to do the job. Without it, you’d probably notice streaking and residue left behind. In my experience, I’ve noticed that creams and soaps that skip stabilizers like this don’t last as long or leave more gunk when rinsed away.
Chemical safety groups like the Environmental Working Group watch over the impact these substances have on our health. Even though triethanolamine has a strong safety record when handled properly, quality control matters. Manufacturers must limit its concentration to avoid skin and eye irritation. Used responsibly, it makes products better for everyone.
Making Paint and Cement Work Like They Should
Triethanolamine keeps things running smoothly behind the scenes in other industries, too. In paints and coatings, it prevents clumps and keeps everything mixed so that the finish ends up smooth instead of streaky. If you’ve ever stirred a can of old paint and fought to get the lumps out, you understand why these chemical helpers matter. Additionally, in cement production, triethanolamine steps in as a grinding aid. It keeps the cement particles apart so the powder remains fine and easy to handle. This quality saves manufacturers money and improves the final product’s strength.
The Push for Transparency and Safer Alternatives
As consumers and regulators push for cleaner, safer products, the demand for transparency grows. Years ago, nobody cared what went into floor polish or deodorant. Now, people want to know the source and safety of every ingredient in things they buy. Modern guidelines, such as those from the Cosmetic Ingredient Review panel, shape how much of substances like triethanolamine makes it into formulas.
The future of formulation pushes manufacturers to consider alternatives and continuous safety reviews. Until then, triethanolamine quietly keeps everything from moisturizers to masonry running smoothly, doing its work in the background and making modern life a lot less messy.
What Is Triethanolamine and Why Does It Show Up Everywhere?
Pick up a random bottle of moisturizer or face wash and there’s a good shot you’ll see triethanolamine on the label. This chemical helps keep creams from separating and adjusts the pH, making them nicer to apply. It’s been floating around the cosmetics world since the early twentieth century, so it’s not some trendy newcomer.
How Brands Use It, and Why People Raise Eyebrows
Triethanolamine pops up in all kinds of products, from shampoos to sunscreens. It helps blend oil and water—a sort of matchmaker for ingredients. Companies like it because it’s cheap and does the job. But with chemical names that long, shoppers get suspicious. It’s normal to wonder if this stuff is safe for regular use.
What the Science Says About Safety
The U.S. FDA keeps an eye on ingredients like this. Triethanolamine, in small amounts (up to 5%), gets a green light in most wash-off products. Research from health agencies shows it rarely causes skin issues, except in people with sensitive or broken skin. Medical studies show allergic reactions are pretty rare. No one wants itchy rashes, and I’ve seen some folks with eczema complain about stinging. Still, for the majority, daily use in cleansers and creams goes unnoticed.
Concerns get louder about “nitrosamines”—bad actors that might form when triethanolamine mixes with some preservatives. Lab tests found nitrosamines can be linked to cancer, but normal cosmetic use doesn’t stack up enough of a risk. Reputable brands try to keep these extra chemicals out. It’s worth giving credit to watchdogs for keeping ingredient levels honest.
My Take from Years of Shopping and Reading Labels
Most people don’t talk about what they put on their skin unless there’s a problem. I’ve spent hours reading ingredient decks, trying to keep breakouts in check. I used to worry about every synthetic-sounding name. Triethanolamine triggered a late-night Google frenzy. Yet, digging deeper made one thing clear: using most mainstream face products with it hasn’t led to big complaints in real life. Dermatologists tell me the same. As with anything, moderation matters and it’s smart to patch test if you’re sensitive.
How to Choose What Goes on Your Skin
Some folks want to avoid anything unpronounceable. There are options out there—brands making products without triethanolamine or similar chemicals. If you prefer natural, those alternatives exist, though texture and shelf life sometimes lack. For others, ease and price matter just as much. Checking for extra certifications or sticking with brands that share their testing results gives extra peace of mind.
Kids or folks with allergies should ask a pharmacist or dermatologist. People with healthy skin who use well-known products usually don’t feel any effects from triethanolamine. It’s not a miracle worker, but it doesn’t lurk as a silent danger for most shoppers, either. If in doubt, ask. Better informed means less stress at the drugstore and more time enjoying life, not worrying about every ingredient.
Understanding What’s in Everyday Products
Triethanolamine shows up on the back of shampoos, face washes, sunscreens, and even printing inks. It’s easy to look past that complicated name, tucked between friendlier ingredients like aloe or vitamin E. Some use it to balance pH or as an emulsifier. It’s a common sight in everything from beauty routines to cleaning up after dinner. People want to know: does it bring any real risk?
Short-Term Contact: What Most Folks Notice
A person might feel an itch or notice redness after using a product with triethanolamine. That’s often the skin’s way of protesting. Those with sensitive skin, eczema, or history of allergies feel it more keenly than others. Using a lotion, for example, with a concentration over 5% can sometimes lead to rashes. Some people have shared stories of eye stinging after using certain shampoos. Washing the area with cool water tends to help, but the discomfort can put you off a product for good.
Long-Term Risks: Reading Studies and Living Carefully
I remember a friend who deeply researched every ingredient before buying any cream. She flagged triethanolamine because there’s talk about it being a possible irritant, and, with repeated use on broken or inflamed skin, it may cause “contact dermatitis.” That means redness, scaling, or tiny blisters. Major health organizations do set safe limits: the FDA watches the levels in cosmetics, and the Scientific Committee on Consumer Safety in Europe capped the concentration at 2.5% for leave-on skin products. They did this for a reason—there’s no shortage of anecdotal complaints popping up on forums and social media.
Concerns Over Nitrosamines
One fact not everybody knows: triethanolamine can react with certain preservatives or other ingredients, forming nitrosamines. Some nitrosamines have been found to cause cancer in lab animals. Trusted bodies like the International Agency for Research on Cancer share concern about repeated, high exposure. Here, it’s less about one night of heavy lotion and more about daily, long-term use at higher-than-recommended concentrations. People worry about the mix, especially if they’re using several products with similar ingredients every day. Manufacturers often test and adjust formulas to keep consumers safe, but stricter labeling would help build trust.
What Helps: Smart Shopping and Skin Awareness
Checking ingredient lists brings peace of mind. If you deal with allergies, sensitive skin, or eczema, brands that leave out triethanolamine or keep concentrations low usually work better. Patch testing—the classic dab-and-wait—really does pay off. Sharing real-life reactions with consumer safety agencies can spark reviews and tighter rules. Medical professionals like dermatologists should always get a say—consulting them brings reliable advice for ingredient concerns.
Brands choosing “cleaner” alternatives that don’t trigger these side effects tend to win loyalty from picky shoppers. These alternatives reduce risk, and hearing from people who switched products and saw relief reminds us these decisions matter. People deserve honesty and clear guidelines about what they’re putting on their bodies.
Understanding Triethanolamine’s Presence
Triethanolamine, or TEA, pops up in plenty of products around us. I’ve seen it on the back of shampoo bottles, in laundry detergents, and even in some weed-control sprays at the hardware store. Formulators like TEA for its smooth blending with water and oils, helping everything mix without separating. This ubiquity leads to one key reality: what goes down the drain eventually finds its way into our water systems.
Breaking Down the Risks
A lot of folks worry about chemicals like TEA getting into rivers and lakes. This concern isn’t unfounded. TEA doesn’t break down quickly. It may stick around long enough to affect aquatic life, especially fish and algae. Some studies point out that in large enough doses, TEA disrupts cell membranes in small aquatic animals. Authorities like the EPA and European Chemicals Agency have flagged it for careful watch, since accumulation poses a longer-term risk rather than instant toxicity.
My background in local watershed protection taught me that bioaccumulation often flies under people’s radar until obvious damage shows up. For example, foam insulation makers and car-care cleaning products often discharge wastewater containing TEA. With enough repeated use across neighborhoods and factories, even smaller leaks into waterways add up.
The Facts: TEA, Water, and Soil
TEA dissolves well in water, which isn’t always good news for nature. Once in a stream or pond, the chemical moves along currents and can travel quite far. Soil doesn’t break it down much faster. Since TEA resists rapid decomposition, soil bacteria and sunlight alone won’t knock it out in a hurry.
We’ve seen similar issues with other chemicals — think of the way PCBs spread far beyond where people dumped them years ago. TEA isn’t on the same level as classic persistent pollutants or heavy metals, but ignoring its slow breakdown means letting small quantities linger year after year. Some research even connects trace amounts in urban runoff to minor algae blooms or stressed fish in overburdened waters.
What Can Change?
Plenty of options exist to help limit TEA’s environmental impact. Factories can update their wastewater treatment processes to snatch up more TEA before water flows out. I’ve met engineers who install extra filtration steps and use bacteria that munch on amines like TEA. Municipal water plants could prioritize these upgrades, especially in areas near big manufacturers or dense cities.
Consumers can add their voice by contacting brands and asking about greener alternatives. It’s not about fear-mongering but about demanding safer chemistry. More companies have started seeking plant-based solutions for emulsifying and stabilizing products, and regulatory agencies are pushing to keep up with the science.
Education also goes a long way. If every household cut back on pouring unnecessary chemicals down the drain, the sum of those small actions brings a measurable change. Community clean-ups and support for local water quality programs help remind everyone what’s at stake. TEA alone won’t tip the scales, but it serves as one more reason to keep fighting for smarter, cleaner production and disposal all around us.
What's Triethanolamine Doing in Everyday Hair Care?
Triethanolamine, or TEA, pops up a lot on ingredient lists for gels, mousses, and shampoos. Most chemists use it as a pH balancer and an emulsifier. That means it helps recipes stay creamy or foamy—especially if water and oil need to mix. If you ever tried to make mayonnaise from scratch, you probably saw how tough it gets without a go-between. Same thing here. But just because science can add it, doesn’t always mean it should go in everything without a second thought.
Health and Safety: What Do We Really Know?
Plenty of people check ingredient labels for good reason. Safety rules in places like the United States and Europe allow TEA in low amounts: under 5% by weight, according to regulations. When used as instructed, TEA rarely irritates skin. Still, the risk isn’t zero. Cosmetic safety reviews show high concentrations or long-term contact sometimes spark allergic reactions. Some studies hint that TEA could form nitrosamines if it mixes with certain preservatives, especially nitrites. Most nitrosamines are classified as possible human carcinogens.
To be clear, common products use TEA in such small doses, it would take regular, heavy exposure to trigger most issues scientists worry about. But the key lesson: mixing TEA with certain chemicals isn’t wise, and no one likes gambling with personal health. A quick glance at ingredient lists can keep you safer than hoping rules catch every problem before shelves fill.
Personal Experience: Trusting What You Can’t See?
I grew up in a house where we’d share whatever shampoo was on sale. Many times it left my scalp feeling itchy. As I got older and started reading up on what actually goes into these bottles, I noticed products with TEA near the top sometimes triggered irritation more than others. It doesn’t affect everyone, but for people dealing with eczema, psoriasis, or just sensitive skin, even low levels might bring trouble.
Friends who switched to “natural” or sulfate-free lines usually report less burning or flaking. Sometimes these cleaner formulas skip TEA altogether. That’s not proof against it—just a pattern I’ve seen time and time again.
What Goes Into a Safer Option?
There’s no shortage of alternatives. Manufacturers use things like citric acid or sodium citrate for pH adjustments, and plant-based emulsifiers from lecithin or coconut oil. The move toward gentler ingredients gains momentum every year. A big reason: consumers push for transparency and products that feel good after long-term use, not just for the first few washes.
You can spot safer choices by scanning for shorter ingredient lists, or looking for assurances from third-party groups like EWG or dermatologist testing. If your routine product causes burning, redness, or dryness, try a patch test or reach out to a dermatologist. Pressing companies for more open labeling pushes the whole industry further in the right direction.
Taking Control of What Touches Your Skin
The bottom line? TEA in small amounts does its job, and scientists rate it as low risk in typical hair items. But anyone can react differently, especially with repeat exposure or allergies. Study up on what goes into your products, watch your body’s response, and don’t be afraid to ask questions. Your scalp’s health affects your day-to-day comfort as much as that “fresh salon feel” does.
| Names | |
| Preferred IUPAC name | 2,2',2''-nitrilotriethanol |
| Other names |
TEA
Trolamine Triethylolamine Tris(2-hydroxyethyl)amine |
| Pronunciation | /traɪˌɛθəˈnɒləmiːn/ |
| Identifiers | |
| CAS Number | 102-71-6 |
| Beilstein Reference | 1209242 |
| ChEBI | CHEBI:45814 |
| ChEMBL | CHEMBL1536 |
| ChemSpider | 5817 |
| DrugBank | DB03584 |
| ECHA InfoCard | 03b381af-036a-4154-890a-6b5937b3a888 |
| EC Number | 203-049-8 |
| Gmelin Reference | 8229 |
| KEGG | C14640 |
| MeSH | D014258 |
| PubChem CID | 7340 |
| RTECS number | KL9275000 |
| UNII | AZI07JPJ0Z |
| UN number | UN2499 |
| Properties | |
| Chemical formula | C6H15NO3 |
| Molar mass | 149.188 g/mol |
| Appearance | Colorless to pale yellow viscous liquid |
| Odor | slight ammonia odor |
| Density | 1.124 g/cm³ |
| Solubility in water | Miscible |
| log P | -1.0 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 7.8 |
| Basicity (pKb) | 4.1 |
| Magnetic susceptibility (χ) | -7.3·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.482 |
| Viscosity | Viscosity: 450 mPa·s (at 20°C) |
| Dipole moment | 5.1396 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1153.95 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3930 kJ/mol |
| Pharmacology | |
| ATC code | C05AX17 |
| Hazards | |
| Main hazards | Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | Hazard statement(s): H315, H318 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Flash point | 179 °C |
| Autoignition temperature | 335°C |
| Lethal dose or concentration | LD50 (Oral, Rat): 6400 mg/kg |
| LD50 (median dose) | 4,920 mg/kg (rat, oral) |
| NIOSH | T45 |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | 1000 mg/L |
| IDLH (Immediate danger) | 650 mg/m3 |
| Related compounds | |
| Related compounds |
Diethanolamine
Monoethanolamine Tetraethanolamine |

